Dose sparing enabled by immunization with influenza vaccine using orally dissolving film
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Influenza vaccine delivered by orally dissolving film vaccine (ODFV) is a promising approach. In this study, we generated three ODFVs each comprising pulluan and trehalose with different doses of inactivated A/Puerto Rico/8/34, H1N1 virus (ODFV I, II, III) to evaluate their dose-sparing effect in mice. The ODFVs were placed on the tongues of mice to elicit immunization and after 3 immunizations at 4-week intervals, mice were challenged with a lethal dose of A/PR/8/34 to assess vaccine-induced protection. The 3 ODFVs containing 50, 250, or 750 μg of inactivated viruses elicited virus-specific antibody responses and virus neutralization in a dose-dependent manner. Dose-dependent antibody responses were also observed from the mucosal tissue samples, and also from antibody-secreting cells of the lungs and spleens. ODFV-induced cellular immunity, particularly germinal center B cells and T cells were also dose-dependent. Importantly, all 3 ODFVs evaluated in this study provided complete protection by strongly suppressing the pro-inflammatory cytokine production and lung virus titers. None of the immunized mice underwent noticeable weight loss nor succumbed to death, a phenomenon that was only observed in the infection challenge controls. These results indicated that the protection conferred by a low dose influenza vaccine formulated in ODF is comparable to that of a high-dose vaccine, thereby enabling vaccine dose sparing effect.
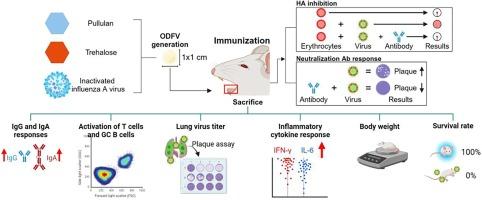
使用口腔溶解膜免疫接种流感疫苗可节省剂量。
通过口腔溶解膜疫苗(ODFV)接种流感疫苗是一种很有前景的方法。在这项研究中,我们生成了三种口服溶膜疫苗(ODFV I、II、III),每种疫苗都含有不同剂量的灭活甲型/波多黎各/8/34甲型 H1N1 流感病毒(A/Puerto Rico/8/34, H1N1 virus)和拉布脲(pulluan)和曲哈洛糖(trehalose),以评估它们对小鼠的剂量节省效果。将 ODFV 放在小鼠舌头上诱导免疫,间隔 4 周进行 3 次免疫后,用致死剂量的 A/PR/8/34 对小鼠进行挑战,以评估疫苗诱导的保护作用。含有 50、250 或 750 μg 灭活病毒的 3 种 ODFV 以剂量依赖的方式引起病毒特异性抗体反应和病毒中和。从粘膜组织样本以及肺和脾的抗体分泌细胞中也观察到了剂量依赖性抗体反应。ODFV诱导的细胞免疫,特别是生殖中心B细胞和T细胞也呈剂量依赖性。重要的是,本研究中评估的所有 3 种 ODFV 都能强烈抑制促炎细胞因子的产生和肺部病毒滴度,从而提供完全的保护。没有一只免疫小鼠出现明显的体重减轻或死亡,而这种现象只有在感染挑战对照组中才能观察到。这些结果表明,用 ODF 配制的低剂量流感疫苗可提供与高剂量疫苗相当的保护,从而实现疫苗剂量节省的效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


