A conserved sequence that sparked the field of evo-devo
IF 2.5
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
Abstract
The discovery that homeotic genes in Drosophila are conserved and utilized for embryonic development throughout the animal kingdom, including humans, revolutionized the fields of developmental biology and evolutionary developmental biology (evo-devo). In a pair of back-to-back papers published in Cell in 1984, researchers at the Biozentrum in Basel, Switzerland, showed that the homeobox – previously identified as a sequence shared by homeotic genes in Drosophila – was also present in the genome of diverse animals. The first paper (McGinnis et al., 1984a) showed that genomes of both invertebrates and vertebrates contain sequences that cross-hybridized with Drosophila homeobox probes. The second paper (Carrasco et al., 1984) identified a cross-hybridizing sequence in the model system Xenopus laevis. They then isolated the first vertebrate homeobox-containing gene by cloning and sequencing of the corresponding genomic region. Finally, they showed that this gene (AC1, later renamed HoxC6) was expressed during embryonic development, the first evidence that developmentally expressed Drosophila genes could be used to isolate regulators of vertebrate embryonic development. These findings led to a flurry of activity in the evo-devo field, initially focused on isolating Hox genes across diverse species, and then expanding to isolation of other gene families based on Drosophila orthologs, an approach that continues today. This led to the notion of a conserved genetic toolkit for embryonic development, currently accepted, but unexpected at the time of its discovery. We attempt to provide some context for the sea-change in thinking that these discoveries brought about by referring to Jean Piaget's theories about the sequential acquisition of scientific knowledge.
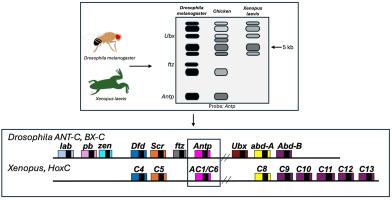
一个保守序列引发了进化-变形领域的研究。
果蝇的同源基因在包括人类在内的整个动物界的胚胎发育过程中都得到了保留和利用,这一发现彻底改变了发育生物学和进化发育生物学(evo-devo)领域。瑞士巴塞尔生物中心(Biozentrum in Basel)的研究人员在 1984 年连续发表在《细胞》(Cell)杂志上的两篇论文中表明,同源染色体(homeobox)--以前被确定为果蝇同源基因共有的序列--也存在于各种动物的基因组中。第一篇论文(McGinnis 等人,1984a)表明,无脊椎动物和脊椎动物的基因组都含有与果蝇同源基因探针交叉杂交的序列。第二篇论文(Carrasco 等人,1984 年)在模式系统爪蟾中发现了交叉杂交序列。随后,他们通过对相应基因组区域的克隆和测序,分离出第一个脊椎动物含同源框基因。最后,他们发现该基因(AC1,后更名为 HoxC6)在胚胎发育过程中表达,首次证明果蝇发育表达的基因可用于分离脊椎动物胚胎发育的调控因子。这些发现引发了进化-变形领域的一系列活动,最初的重点是分离不同物种的 Hox 基因,然后扩展到根据果蝇的直向同源物分离其他基因家族,这种方法一直延续至今。这导致了胚胎发育保守基因工具包的概念,这一概念目前已被接受,但在发现时却出乎意料。我们试图通过引用让-皮亚杰(Jean Piaget)关于科学知识循序获取的理论,为这些发现所带来的思维巨变提供一些背景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


