Regulatory T cells engineered with polyphenol-functionalized immunosuppressant nanocomplexes for rebuilding periodontal hard tissue under inflammation-challenged microenvironment
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Global aging heightens the risk of oral disorders, among which periodontitis is the major cause of tooth loss in the aging population. The regeneration of damaged periodontal hard tissue is highly challenging due to the existence of the refractory local inflammation. Owing to the potent anti-inflammatory capabilities, regulatory T cells hold great promise in immunotherapies for tissue regeneration. However, the transferred regulatory T cells can alter their phenotypes and functions in local inflammatory milieu, significantly impairing their therapeutic efficacy. Herein, we introduce a novel regulatory T cell-based nanobiohybrid system bearing polyphenol-functionalized rapamycin nanocomplexes. The sustained in situ release of immunosuppressant rapamycin from the cell-attached nanocomplexes maintains the anti-inflammatory phenotype of regulatory T cells in the inflammatory milieu. The synergistic actions of the anti-inflammatory cytokines secreted and the immunosuppressant released guide a pro-resolving polarization of macrophages and enhance osteogenic differentiation of bone marrow-derived stromal cells. The stabilized phenotype of the regulatory T cells dramatically promoted the resolution of periodontal inflammation and the repair of the hard tissue (alveolar bone) in vivo. Overall, these studies highlight a potent regulatory T cell-based nanobiohybrid therapy to treat periodontitis by modulating periodontal immune microenvironment.
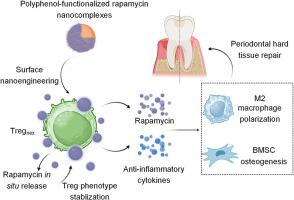
利用多酚功能化免疫抑制剂纳米复合物设计的调节性 T 细胞,在炎症挑战的微环境下重建牙周硬组织。
全球老龄化加剧了口腔疾病的风险,其中牙周炎是老龄人口牙齿脱落的主要原因。由于存在难治性局部炎症,受损牙周硬组织的再生极具挑战性。由于调节性 T 细胞具有强大的抗炎能力,因此在组织再生的免疫疗法中大有可为。然而,转入的调节性 T 细胞在局部炎症环境中会改变其表型和功能,从而严重影响其疗效。在这里,我们介绍了一种基于调节性 T 细胞的新型纳米生物杂交系统,该系统含有多酚功能化雷帕霉素纳米复合物。免疫抑制剂雷帕霉素从细胞附着的纳米复合物中持续原位释放,从而维持了调节性 T 细胞在炎症环境中的抗炎表型。分泌的抗炎细胞因子和释放的免疫抑制剂协同作用,引导巨噬细胞向有利于溶解的极化方向发展,并增强骨髓基质细胞的成骨分化。表型稳定的调节性 T 细胞极大地促进了牙周炎症的缓解和体内硬组织(牙槽骨)的修复。总之,这些研究强调了通过调节牙周免疫微环境来治疗牙周炎的一种有效的基于调节性 T 细胞的纳米生物混合疗法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


