Effects of sulforaphane on prostate cancer stem cells-like properties: In vitro and molecular docking studies
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The increasing incidence of prostate cancer worldwide has spurred research into novel therapeutics for its treatment and prevention. A critical factor contributing to its incidence and development is the presence of prostate cancer stem cells (PCSCs). Targeting PCSCs has become key in enhancing therapeutic and clinical outcomes of prostate cancer. Sulforaphane (SFN), a compound found in cruciferous vegetables, has shown effective antineoplastic activity in prostate cancer. Yet, its mechanisms of action in PCSCs remains unclear. In the present study, tumorsphere formation assay was used to isolate and enrich PCSCs from PC-3 cells. Our results found that SFN effectively reduced the activity of PCSCs, including the ability of tumorsphere formation, the number of CD133 positive cells, and the expression of PCSCs markers. Moreover, the data showed that SFN inhibited PCSCs through downregulating the activation of Wnt/β-catenin and hedgehog signaling pathways in PCSCs. Furthermore, the verification experiments showed that the activators of Wnt/β-catenin (LiCl) and hedgehog (purmorphamine) attenuated the effects of SFN on PCSCs, including the expression of stem cell markers, cell proliferation and apoptosis. Meanwhile, suppression of β-catenin or Smoothened enhanced the effects of SFN on PCSCs. In addition, molecular docking further indicated that SFN inhibited Wnt/β-catenin and hedgehog pathways by directly targeting β-catenin and Smoothened. Taken together, our results demonstrated that SFN targeted PCSCs through Wnt/β-catenin and hedgehog pathways to inhibit stemness and proliferation and induce apoptosis. Findings from this study could provide new insights into SFN as a dietary supplement or adjunct to chemotherapy.
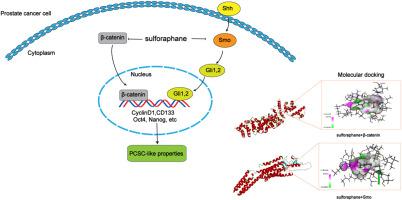
莱菔硫烷对前列腺癌干细胞类特性的影响:体外和分子对接研究。
随着全球前列腺癌发病率的不断上升,促进了对治疗和预防前列腺癌的新型疗法的研究。导致前列腺癌发病和发展的一个关键因素是前列腺癌干细胞(PCSCs)的存在。靶向 PCSCs 已成为提高前列腺癌治疗和临床疗效的关键。在十字花科蔬菜中发现的一种化合物--莱菔硫烷(SFN)对前列腺癌具有有效的抗肿瘤活性。然而,它在 PCSCs 中的作用机制仍不清楚。本研究采用瘤球形成试验从PC-3细胞中分离并富集PCSCs。结果发现,SFN 能有效降低 PCSCs 的活性,包括肿瘤球的形成能力、CD133 阳性细胞的数量以及 PCSCs 标志物的表达。此外,数据还显示,SFN通过下调PCSCs中Wnt/β-catenin和刺猬信号通路的激活来抑制PCSCs。此外,验证实验表明,Wnt/β-catenin(氯化锂)和hedgehog(嘌呤吗啉)的激活剂削弱了SFN对PCSCs的影响,包括干细胞标志物的表达、细胞增殖和凋亡。同时,β-catenin或Smoothened的抑制增强了SFN对PCSCs的影响。此外,分子对接进一步表明,SFN通过直接靶向β-catenin和Smoothened抑制了Wnt/β-catenin和刺猬通路。综上所述,我们的研究结果表明,SFN通过Wnt/β-catenin和刺猬通路靶向PCSCs,抑制干性和增殖并诱导凋亡。这项研究的结果可为SFN作为膳食补充剂或化疗辅助药物提供新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


