Synthesis, biological evaluation and mechanism study of a novel indole-pyridine chalcone derivative as antiproliferative agent against tumor cells through dual targeting tubulin and HK2
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Chalcones have the characteristics of simple structure, easy synthesis and potent anti-tumor activity. Herein, a small library of fifty-five novel indole-chalcone derivatives were rationally designed and facilely synthesized. Consequently, their antiproliferative activity was systematically evaluated. Among which, compound 26 exhibited the most potent antiproliferative activity, with IC50 value of 0.764 μM against MD-MBA-231 cells. Moreover, it displayed a 5-fold selectivity compared with normal human cells. Further investigation revealed that compound 26 bound at the colchicine binding site of tubulin, disrupted their fibrous structure, thereby blocking the progression of the cell cycle and inducing apoptosis. Molecular docking and cellular thermal shift assay (CETSA) experiments further demonstrated that compound 26 could specifically bind to hexokinase 2 (HK2) and inhibit its activity, leading to impaired mitochondrial function and hindered mitochondrial respiration. Based on the quantitative structure-activity relationship study, further structure modifications were performed. Employing biotin probe pull-down assays, we demonstrated that compound 26 exerted its antiproliferative activity through a dual targeting mechanism, which simultaneously disrupted microtubule function and inhibited HK2 activity. Taken together, these results highlighted that compound 26 might be a promising antiproliferative agent for human cancer therapy.
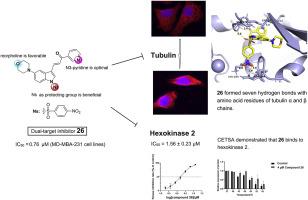
一种新型吲哚吡啶查尔酮衍生物的合成、生物学评价和机理研究:通过双重靶向小管蛋白和 HK2 抗肿瘤细胞增殖剂
查耳酮具有结构简单、易于合成、抗肿瘤活性强等特点。在此,我们合理地设计并简便地合成了一个由 55 种新型吲哚-查尔酮衍生物组成的小型文库。随后,对它们的抗肿瘤活性进行了系统评估。其中,化合物 26 对 MD-MBA-231 细胞的抗增殖活性最强,IC50 值为 0.764 μM。此外,与正常人细胞相比,它还具有 5 倍的选择性。进一步研究发现,化合物 26 与小管蛋白的秋水仙碱结合位点结合,破坏了小管蛋白的纤维结构,从而阻断了细胞周期的进展并诱导细胞凋亡。分子对接和细胞热转移实验(CETSA)进一步证明,化合物 26 能与己糖激酶 2(HK2)特异性结合并抑制其活性,从而导致线粒体功能受损和线粒体呼吸受阻。在定量结构-活性关系研究的基础上,对化合物进行了进一步的结构修饰。通过生物素探针牵引实验,我们证明化合物 26 通过双重靶向机制发挥其抗增殖活性,即同时破坏微管功能和抑制 HK2 活性。综上所述,这些结果表明,化合物 26 可能是一种很有前途的人类癌症治疗抗增殖剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


