Nanosheet Iron Phosphate by an Efficient Route for LiFePO4 Cathode Material
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
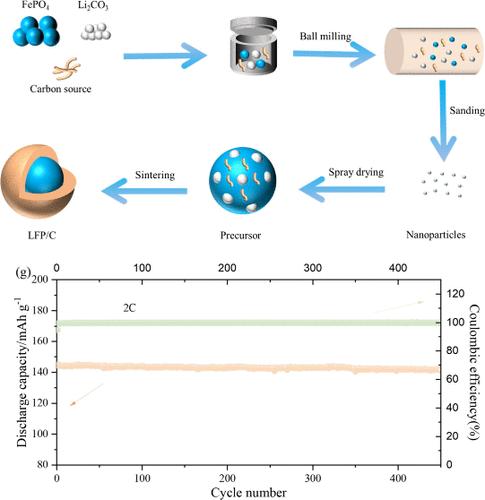
The precursor of FePO4·2H2O is prepared by a liquid phase conversion method with low-cost fine Fe3O4 ore powder from magnetite flotation, and the LiFePO4/C is synthesized by sanding and spray followed by roasting technology. By controlling the excess coefficient of phosphate to Fe, the influence on the degree of chemical reaction and the preferred orientation of the precursor of nano sheet FePO4·2H2O on the (020) face is investigated. The results indicate that when the mole of phosphate is 2.5 times that of iron, the thickness of the FePO4·2H2O precursor nanosheet is the thinnest, resulting in the synthesis of LiFePO4/C materials with the smallest primary particles and the best electrochemical properties. It can be observed that the specific discharge capacity of the as-prepared LiFePO4/C can reach 150.5 mAh/g at 1 C, and the capacity retention rate is still over 96% after 450 cycles at 2 C. At the same time, the Re-LFP/C-2 synthesized with FePO4·2H2O by recycling H3PO4 mother liquor can achieve the same excellent electrochemical performance as the LFP/C-2 synthesized with fresh H3PO4. It is demonstrated that this route has promising development prospects and is easily scalable. At the same time, this synthetic route is cheaper to synthesize and produces less wastewater, which provides a basis for exploring the green, efficient, and low-cost synthesis route of LiFePO4/C.
用高效方法制备磷酸铁锂离子阴极材料的纳米片状磷酸铁
以磁铁矿浮选得到的低成本精细Fe3O4矿粉为原料,通过液相转化法制备FePO4-2H2O前驱体,并采用砂磨喷涂后焙烧技术合成LiFePO4/C。通过控制磷酸盐与铁的过量系数,研究了化学反应程度和纳米片状 FePO4-2H2O 前驱体在(020)面上的优先取向的影响。结果表明,当磷酸盐的摩尔数是铁摩尔数的 2.5 倍时,FePO4-2H2O 前驱体纳米片的厚度最薄,因此合成的 LiFePO4/C 材料的原生颗粒最小,电化学性能最好。同时,通过回收利用 H3PO4 母液与 FePO4-2H2O 合成的 Re-LFP/C-2 与用新鲜 H3PO4 合成的 LFP/C-2 一样具有优异的电化学性能。研究表明,这条路线具有广阔的发展前景,而且易于推广。同时,该合成路线合成成本较低,产生的废水较少,为探索绿色、高效、低成本的磷酸铁锂/C合成路线提供了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


