ZnO‐CuS/F127 Hydrogels with Multienzyme Properties for Implant‐Related Infection Therapy by Inhibiting Bacterial Arginine Biosynthesis and Promoting Tissue Repair
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Implant‐related infections are characterized by the formation of bacterial biofilms. Current treatments have various drawbacks. Nanozymes with enzyme‐like activity can produce highly toxic substances to kill bacteria and remove biofilms without inducing drug resistance. However, it is difficult for current monometallic nanozymes to function well in complex biofilm environments. Therefore, the development of multimetallic nanozymes with efficient multienzyme activities is crucial. In the present study, bimetallic nanozyme, ZnO‐CuS nanoflowers with peroxidase (POD), glutathione oxidase (GSH‐Px), and catalase (CAT) activity are successfully synthesized via calcination and loaded into F127 hydrogels for the treatment of implant‐related infections. The ability of ZnO‐CuS nanoflowers to bind bacteria is key for efficient antimicrobial activity. In addition, ZnO‐CuS nanoflowers with H
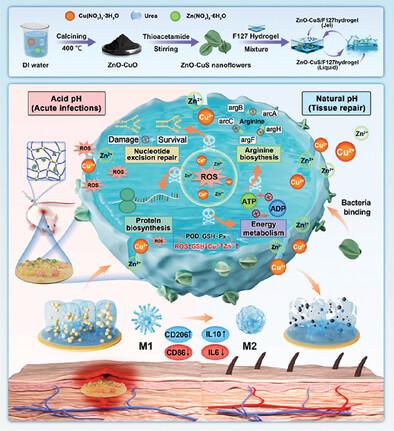
具有多酶特性的 ZnO-CuS/F127 水凝胶可通过抑制细菌精氨酸合成和促进组织修复来治疗与种植体有关的感染
与种植体相关的感染以细菌生物膜的形成为特征。目前的治疗方法存在各种弊端。具有类似酶活性的纳米酶可以产生剧毒物质,杀死细菌并清除生物膜,同时不会诱发耐药性。然而,目前的单金属纳米酶很难在复杂的生物膜环境中发挥良好作用。因此,开发具有高效多酶活性的多金属纳米酶至关重要。在本研究中,通过煅烧成功合成了具有过氧化物酶(POD)、谷胱甘肽氧化酶(GSH-Px)和过氧化氢酶(CAT)活性的双金属纳米酶 ZnO-CuS纳米花,并将其装载到 F127 水凝胶中,用于治疗与种植体相关的感染。ZnO-CuS 纳米花结合细菌的能力是高效抗菌的关键。此外,含有 H2O2 的 ZnO-CuS 纳米花会破坏 MRSA 的新陈代谢,包括精氨酸合成、核苷酸切除修复、能量代谢和蛋白质合成。实验证明,ZnO-CuS/F127 水凝胶与 H2O2 结合使用可有效清除生物膜感染,并促进 M1 型巨噬细胞向 M2 修复表型巨噬细胞转换,从而治疗小鼠的植入感染。此外,ZnO-CuS/F127 水凝胶还具有良好的生物安全性,其毒性可忽略不计。ZnO-CuS/F127 水凝胶为植入物相关感染的愈合提供了一种前景广阔的生物医学策略,凸显了双金属纳米酶在临床应用方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


