Polyesterase activity is widespread in the family IV carboxylesterases from bacteria
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Enzyme-based depolymerization of plastics, including polyesters, has emerged as a promising approach for plastic waste recycling and reducing environmental plastic pollution. Currently, most of the known polyester-degrading enzymes are represented by a few natural and engineered PETases from the carboxylesterase family V. To identify novel groups of polyesterases, we selected 25 proteins from the carboxylesterase family IV, which share 22 % to 80 % sequence identity to the metagenomic thermophilic polyesterase IS12. All purified proteins were found to be active against chromogenic para-nitrophenyl esters with a preference for short acyl chains. Screening for polyesterase activity using emulsified polyesters demonstrated the presence of hydrolytic activity against bis(benzoyloxyethyl) terephthalate (3PET), polycaprolactone (PCL), and polylactic acid (PLA) in all tested proteins. Biochemical characterization of four selected polyesterases revealed high thermostability in CBA10055, whereas the mesophilic GEN0105 exhibited higher polyesterase activity. Two ancestral variants of GEN0105 showed higher thermostability and activity against PCL and PLA, but reduced activity with amorphous PET. Furthermore, six established PETases were found to be highly active against PCL and PLA. Thus, our results indicate that polyesterase activity is widespread in the family IV carboxylesterases, and that most polyesterases are promiscuous being able to degrade different polyesters.
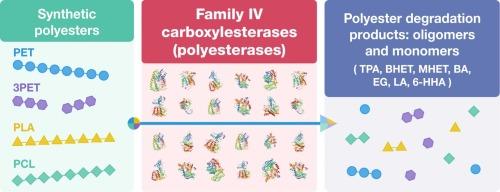
细菌中的羧基酯酶家族 IV 普遍具有聚酯酶活性
以酶为基础的塑料(包括聚酯)解聚已成为塑料废物回收利用和减少环境塑料污染的一种有前途的方法。目前,大多数已知的聚酯降解酶都是以羧基酯酶家族 V 中的几种天然和工程 PET 酶为代表的。为了确定新的聚酯酶群,我们从羧基酯酶家族 IV 中挑选了 25 种蛋白质,它们与元基因组嗜热聚酯酶 IS12 有 22% 至 80% 的序列相同性。研究发现,所有纯化的蛋白质都对发色性对硝基苯酯具有活性,并偏好短酰基链。利用乳化聚酯进行的聚酯酶活性筛选表明,所有测试蛋白质都具有水解对苯二酸二(苯甲酰氧乙基)酯(3PET)、聚己内酯(PCL)和聚乳酸(PLA)的活性。四种所选聚酯酶的生化特征表明,CBA10055 具有很高的热稳定性,而中嗜性 GEN0105 则表现出更高的聚酯酶活性。GEN0105 的两个祖先变体对 PCL 和聚乳酸表现出更高的热稳定性和活性,但对无定形 PET 的活性降低。此外,还发现六种已建立的 PET 酶对 PCL 和 PLA 具有较高的活性。因此,我们的研究结果表明,聚酯酶活性广泛存在于羧基酯酶家族 IV 中,而且大多数聚酯酶具有杂合性,能够降解不同的聚酯。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


