Constructing Angstrom-Level Ion Pocket Array in 1D Channel Wall for Efficient Lithium Ion Sieving
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The rapid development of new energy industry is leading to the scarcity of lithium (Li) metal. Rational design of adsorbents for efficient separation of Li+ ion from aqueous media is pivotal to solve the recovery of this valuable resource. Current adsorbents generally suffer from the drawbacks in adsorption capacity, kinetics, and selectivity. Herein, a novel and ultra-stable metal–organic framework is designed for Li+ separation. The dense oxygen atoms on the cambered wall of its 1D channel encircle to form angstrom-level tetrahedral ion pockets array, acting as the dominant adsorption sites. This rational distribution of the array avoids the pore blockage caused by the pre-adsorbed ions, thereby accelerating the diffusion of subsequent ions into the interior pore. Meanwhile, this tetrahedral pocket shows distinct electronegativity and strong chelation effect for Li+. Benefiting from these specifics, this adsorbent exhibits a record-breaking adsorption capacity for Li+ (76.1 mg g−1) and short equilibrium time (30 min). Moreover, the selective adsorption of Li+ over Na+, K+, Ca2+, and Mg2+ is achieved due to the matched Li+ ion diameter with the pocket/channel sizes and lower energy barrier for dehydration. Thus, this work proposes a feasible strategy for the construction of novel MOFs for ions adsorption.
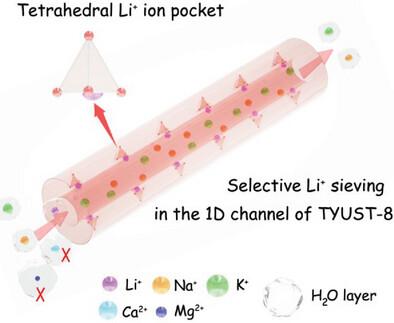
在一维通道壁中构建埃级离子袋阵列,实现高效锂离子筛分
新能源产业的快速发展导致金属锂(Li)的稀缺。合理设计从水介质中高效分离 Li+ 离子的吸附剂是解决这一宝贵资源回收问题的关键。目前的吸附剂普遍存在吸附容量、动力学和选择性方面的缺陷。本文设计了一种用于分离 Li+ 的新型超稳定金属有机框架。其一维通道凸壁上的致密氧原子环绕形成埃级四面体离子袋阵列,成为主要的吸附位点。这种阵列的合理分布避免了预吸附离子造成的孔隙堵塞,从而加速了后续离子向内部孔隙的扩散。同时,这个四面体口袋对 Li+ 具有明显的电负性和较强的螯合作用。得益于这些特性,这种吸附剂对 Li+ 的吸附容量(76.1 mg g-1)和较短的平衡时间(30 分钟)均打破了记录。此外,由于 Li+ 离子直径与口袋/通道尺寸相匹配,且脱水能垒较低,因此实现了 Li+ 对 Na+、K+、Ca2+ 和 Mg2+ 的选择性吸附。因此,这项工作为构建新型离子吸附 MOFs 提出了一种可行的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


