Probing the Interplay of Protein Self‐Assembly and Covalent Bond Formation in Photo‐Crosslinked Silk Fibroin Hydrogels
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
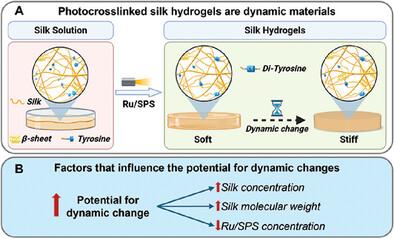
Covalent crosslinking of silk fibroin via native tyrosine residues has been extensively explored; however, while these materials are very promising for biomedical, optical, soft robotics, and sensor applications, their structure and mechanical properties are unstable over time. This instability results in spontaneous silk self‐assembly and stiffening over time, a process that is poorly understood. This study investigates the interplay between self‐assembly and di‐tyrosine bond formation in silk hydrogels photo‐crosslinked using ruthenium (Ru) and sodium persulfate (SPS) with visible light. The effects of silk concentration, molecular weight, Ru/SPS concentration, and solvent conditions are examined. The Ru/SPS system enables rapid crosslinking, achieving gelation within seconds and incorporating over 90% of silk into the network, even at very low protein concentrations (≥0.75% wt/v). A model emerges where silk self‐assembly both before and after crosslinking affects protein phase separation, mesoscale structure, and dynamic changes in the hydrogel network over time. Silk concentration has the greatest impact on hydrogel properties, with higher silk concentration hydrogels experiencing two orders of magnitude increase in stiffness within 1 week. This new understanding and ability to tune hydrogel properties and dynamic stiffening aids in developing advanced materials for 4D biofabrication, sensing, 3D cancer models, drug delivery, and soft robotics.
探究光交联蚕丝纤维素水凝胶中蛋白质自组装与共价键形成的相互作用
通过原生酪氨酸残基对蚕丝纤维素进行共价交联的研究已得到广泛探索;然而,虽然这些材料在生物医学、光学、软机器人和传感器应用方面前景广阔,但其结构和机械性能却长期不稳定。这种不稳定性会导致丝的自发自组装和随时间推移而变硬,而人们对这一过程还知之甚少。本研究探讨了在可见光下使用钌(Ru)和过硫酸钠(SPS)光交联的蚕丝水凝胶中自组装和二酪氨酸键形成之间的相互作用。研究了蚕丝浓度、分子量、Ru/SPS 浓度和溶剂条件的影响。Ru/SPS 系统可实现快速交联,在数秒内达到凝胶化,即使在蛋白质浓度很低(≥0.75% wt/v)的情况下,也能将 90% 以上的蚕丝纳入网络。在这个模型中,丝在交联前后的自组装会影响蛋白质相分离、中尺度结构以及水凝胶网络随时间发生的动态变化。蚕丝浓度对水凝胶特性的影响最大,蚕丝浓度越高,水凝胶的硬度在一周内会增加两个数量级。这种新的认识和调整水凝胶特性和动态刚度的能力有助于开发用于 4D 生物制造、传感、3D 癌症模型、药物输送和软机器人技术的先进材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


