Polymetallic sulfide MSx (M = Ni, Fe, and Mo) microspheres for highly selective extraction of Ag+ over Cu2+ and reduction of Ag+ ions to Ag0 metals
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
In this work, through a one-step hydrothermal reaction, MoO42- intercalated NiFe-LDH is obtained, from which a series of multi-metal sulfides of MSx-T (M = Ni, Fe, and Mo, and T refers to sulfuration temperature) are fabricated by sulfuration at different temperature. The NiFeMoS materials present super-fluffy structure with micron spheres assembled by ultrathin nanosheets, resulting in excellent adsorption performance toward silver ions. The optimized NiFeMoS-500 exhibits a maximum Ag(I) capture of 308 mg·g−1 profiting from the hard and soft acid and base (HSAB) affinity and oxidation–reduction (REDOX) reaction. The 10 ppm of Ag+ can be reduced to 0.016 ppm (16 ppb) within 10 min, giving a ∼ 100 % removal. For trace Ag+ (∼ 1 ppm) with a high Cu:Ag ratio of 1000:1, a large separation factor SFAg/Cu (=KdAg/KdCu) of 1.80 × 104 is achieved, and for a more dilute solution of Ag+ (∼ 0.5 ppm) with Cu:Ag ratio of 40:1, a much larger SFAg/Cu of 1.07 × 105 is gained. The S2- in MSx as a soft Lewis base induces the capture of Ag+ as a soft Lewis acid, and the Mo4+ and S2- of MoS2 work as reducing agents to reduce Ag+ ions to Ag0 crystals with morphology of cypress leaves in nanoscale. The Mo4+ can be oxidized to MoO42- which further binds with Ag+ forming Ag2MoO4, thus increasing the silver capture. This work provides inspiration to tailor effective adsorbents for trapping Ag+ from wastewater or extracting noble metals from silver-bearing copper ores leachates.
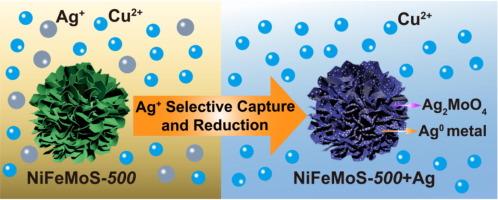
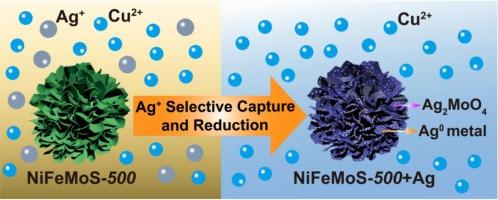
多金属硫化物 MSx(M = Ni、Fe 和 Mo)微球,用于高选择性萃取 Cu2+ 中的 Ag+,并将 Ag+ 离子还原为 Ag0 金属
本研究通过简单的一步水热反应获得了插层镍铁合金的 MoO42-LDH,并在此基础上通过不同温度下的硫化反应制备了一系列 MSx-T(M = 镍、铁和钼,T 指硫化温度)的多金属硫化物。NiFeMoS 材料呈现出由超薄纳米片组装成微米级球体的超蓬松结构,因而对银离子具有优异的吸附性能。通过软硬酸碱(HSAB)亲和力和氧化还原(REDOX)反应,优化后的 NiFeMoS-500 显示出 308 mg-g-1 的最大银离子捕获量。10 ppm 的 Ag+ 可在 10 分钟内降至 0.016 ppm(16 ppb),去除率达 100%。对于痕量 Ag+(≤1 ppm),Cu:Ag 比值高达 1000:1,分离系数 SFAg/Cu (=KdAg/KdCu) 高达 1.80 × 104,而对于更稀释的 Ag+溶液(≤0.5 ppm),Cu:Ag 比值为 40:1,分离系数 SFAg/Cu 高达 1.07 × 105。MSx 中的 S2- 作为软路易斯碱诱导 Ag+ 被软路易斯酸捕获,MoS2 中的 Mo4+ 和 S2- 作为还原剂将 Ag+ 离子还原成具有纳米级柏树叶形态的 Ag0 晶体。Mo4+ 可氧化成 MoO42-,后者进一步与 Ag+ 结合形成 Ag2MoO4,从而增加银的捕获量。这项研究为定制有效的吸附剂提供了灵感,这些吸附剂可吸附废水中的 Ag+,或从含银铜矿浸出液中提取贵金属。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


