Voltage-driven molecular switch of highly periodically N‒heterocyclic adlayer enabled deep cycled zinc metal battery
IF 18.9
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The instable Zn/electrolyte interface due to severe corrosion, especially at high utilization of Zn anode, strongly hindered the practical application of aqueous zinc metal battery. Herein, we report a voltage-driven molecular switch through a reversible transition of the nicotinic molecules between zwitterion and anion to enable deep cycled zinc metal battery. In light of in-situ Raman, the switching mechanism of nicotinic molecules in the electrical double layer is unveiled: during plating, nicotinic molecules switches to zwitterion mode (ON state) with periodical pyridine-ring-substrate adlayer while during stripping, shifts to anion mode with periodical carbonyl group-substrate adlayer (OFF state). The transition of NA molecules enables molecular flipping on the substrate due to the electrostatic force and in this way, in both ON and OFF state, the zinc anode is protected by the adlayer to avoid the zinc corrosion on the anode side. Furthermore, the OTF‒ decomposition is accompanied by the open ring reaction of N‒heterocyclic from nicotinic acid molecules to form highly elastic solid electrolyte interface layer. Benefiting from both the molecular switch function and solid electrolyte interface layer formation, the nicotinic molecules-based electrolyte enables practical zinc metal battery of high energy density (100 Wh/kgelectrode) for over 800 cycles with a cumulative capacity of 2.71 Ah cm−2 at practical condition of low N/P ratio of 2, and lean electrolyte of 10 μL mAh−1, representing the state-of-the-art performance. These findings highlight the utilization of molecular switch and its interfacial protection of the deep cycled zinc anode, and provide a new tactic for the development of high energy metal battery.

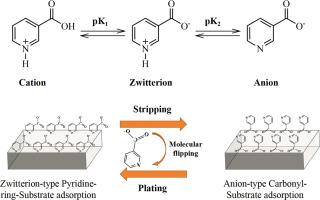
支持深循环锌金属电池的高周期性 N-heterocyclic Adlayer 的电压驱动分子开关
锌/电解质界面因严重腐蚀而不稳定,尤其是在锌阳极利用率较高的情况下,这严重阻碍了锌金属水电池的实际应用。在此,我们报告了一种电压驱动的分子开关,通过烟碱分子在齐聚物和阴离子之间的可逆转换,实现锌金属电池的深循环。根据原位拉曼图,我们揭示了烟碱分子在电双层中的切换机制:在电镀过程中,烟碱分子会切换到具有周期性吡啶环基底吸附层的齐特离子模式(ON 状态);而在剥离过程中,则会切换到具有周期性羰基基底吸附层的阴离子模式(OFF 状态)。NA 分子的转变使分子在静电力的作用下在基底上翻转,这样,在 ON 和 OFF 状态下,锌阳极都受到吸附层的保护,从而避免了阳极侧的锌腐蚀。此外,在 OTF- 分解的同时,烟酸分子中的 N-杂环也会发生开环反应,形成高弹性的固态电解质界面层。得益于分子开关功能和固体电解质界面层的形成,基于烟酸分子的电解质实现了实用锌金属电池的高能量密度(100 Wh/kgelectrode),在低 N/P 比(2)和贫电解质(10 μL mAh-1)的实际条件下,循环次数超过 800 次,累计容量达到 2.71 Ah cm-2,代表了最先进的性能。这些发现凸显了分子开关的利用及其对深循环锌阳极的界面保护,为高能量金属电池的开发提供了新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


