Boosting photocatalytic H2O2 production by incorporating extra electron-donor group over resorcinol-formaldehyde resin
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
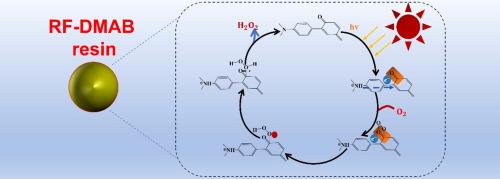
The photocatalytic production of hydrogen peroxide from water and oxygen using solar energy presents a promising approach. Nevertheless, challenges persist regarding the efficiency of this process and the clarification of the underlying catalytic mechanisms. In this study, we synthesized a donor-acceptor type photocatalyst, modified resorcinol-formaldehyde resin. FT-IR and XPS analyses confirmed the successful incorporation of an additional electron donor unit, N, N-dimethylaniline, which interacts with the electron acceptor unit to facilitate the directed migration of photogenerated electrons. Furthermore, this modification creates an electron-rich carbonyl active center, enhancing its capacity to adsorb and reduce O2, thereby improving the photocatalytic production of H2O2. In situ EPR and DRIFT analyses identified key intermediates, substantiating a two-step, single-electron oxygen reduction pathway and providing insights into the catalytic mechanism. Importantly, without the use of sacrificial agents or additional aeration, the photocatalytic production rate of H2O2 reached 22.8 μmol·h−1, underscoring its potential as an effective photocatalyst. This study elucidates the reaction mechanism during the photocatalytic process, offering valuable insights for future material design aimed at enhancing hydrogen peroxide production rates.
在间苯二酚-甲醛树脂上加入额外的电子供体基团,促进光催化 H2O2 的产生
利用太阳能从水和氧气中光催化生产过氧化氢是一种前景广阔的方法。然而,这一过程的效率和潜在催化机理的阐明仍面临挑战。在这项研究中,我们合成了一种供体-受体型光催化剂,即改性间苯二酚-甲醛树脂。傅立叶变换红外光谱(FT-IR)和 XPS 分析证实,该催化剂中成功加入了一个额外的电子供体单元--N, N-二甲基苯胺,它与电子受体单元相互作用,促进了光生电子的定向迁移。此外,这种修饰还产生了一个富含电子的羰基活性中心,增强了其吸附和还原 O2 的能力,从而改善了 H2O2 的光催化生成。原位 EPR 和 DRIFT 分析确定了关键的中间产物,证实了两步单电子氧还原途径,并提供了对催化机理的深入了解。重要的是,在不使用牺牲剂或额外通气的情况下,H2O2 的光催化生产率达到了 22.8 μmol-h-1,这凸显了它作为一种有效光催化剂的潜力。这项研究阐明了光催化过程中的反应机理,为今后设计旨在提高过氧化氢生产率的材料提供了宝贵的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


