Nanomaterial-Mediated Reprogramming of Macrophages to Inhibit Refractory Muscle Fibrosis
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Orofacial muscles are particularly prone to refractory fibrosis after injury, leading to a negative effect on the patient's quality of life and limited therapeutic options. Gaining insights into innate inflammatory response-fibrogenesis homeostasis can aid in the development of new therapeutic strategies for muscle fibrosis. In this study, the crucial role of macrophages is identified in the regulation of orofacial muscle fibrogenesis after injury. Hypothesizing that orchestrating macrophage polarization and functions will be beneficial for fibrosis treatment, nanomaterials are engineered with polyethylenimine functionalization to regulate the macrophage phenotype by capturing negatively charged cell-free nucleic acids (cfNAs). This cationic nanomaterial reduces macrophage-related inflammation in vitr and demonstrates excellent efficacy in preventing orofacial muscle fibrosis in vivo. Single-cell RNA sequencing reveals that the cationic nanomaterial reduces the proportion of profibrotic Gal3+ macrophages through the cfNA-mediated TLR7/9-NF-κB signaling pathway, resulting in a shift in profibrotic fibro-adipogenic progenitors (FAPs) from the matrix-producing Fabp4+ subcluster to the matrix-degrading Igf1+ subcluster. The study highlights a strategy to target innate inflammatory response-fibrogenesis homeostasis and suggests that cationic nanomaterials can be exploited for treating refractory fibrosis.
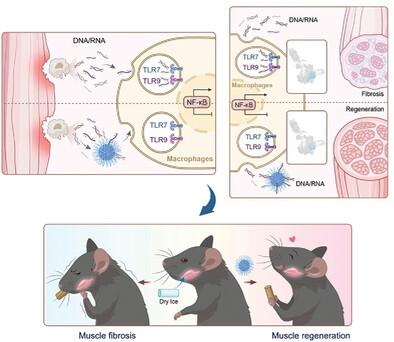
纳米材料介导的巨噬细胞重编程抑制难治性肌肉纤维化
口面部肌肉在受伤后特别容易发生难治性纤维化,从而对患者的生活质量造成负面影响,而且治疗方案有限。深入了解先天性炎症反应-纤维生成的稳态,有助于开发治疗肌肉纤维化的新策略。本研究确定了巨噬细胞在损伤后口面部肌肉纤维生成调控中的关键作用。假设协调巨噬细胞的极化和功能将有利于纤维化的治疗,我们设计了具有聚乙烯亚胺功能化的纳米材料,通过捕获带负电荷的无细胞核酸(cfNAs)来调节巨噬细胞的表型。这种阳离子纳米材料可减少体外与巨噬细胞相关的炎症,并在体内预防口面部肌肉纤维化方面表现出卓越的功效。单细胞RNA测序显示,阳离子纳米材料通过cfNA介导的TLR7/9-NF-κB信号通路降低了嗜碱性Gal3+巨噬细胞的比例,导致嗜碱性纤维脂肪生成祖细胞(FAPs)从产生基质的Fabp4+亚簇转向降解基质的Igf1+亚簇。该研究强调了一种针对先天性炎症反应-纤维生成平衡的策略,并表明阳离子纳米材料可用于治疗难治性纤维化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


