Electrochemical Insights into Hydrogen Peroxide Generation on Carbon Electrodes: Influence of Defects, Oxygen Functional Groups, and Alkali Metals in the Electrolyte
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
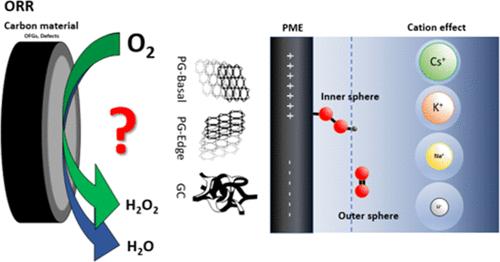
Hydrogen peroxide (H2O2) is an environmentally friendly oxidant, with production reaching 5.7 million tons by 2028 and a market size of USD 4.04 billion by 2029. Understanding the mechanism of oxygen reduction to H2O2 and the structure–activity relations on carbon materials is, therefore, of high significance for the more environmentally friendly synthesis of this important chemical. We have used oriented pyrolytic graphite (PG-edge and PG-basal) and glassy carbon (GC) as model electrodes to investigate the influence of carbon defects, oxygen-containing functional groups, and the presence of alkali metals on the activity and selectivity toward H2O2 production under acidic conditions. Electrochemical measurements, such as rotating ring disk electrode and electrochemical impedance spectroscopy, as well as in situ Raman spectroelectrochemistry indicated that PG-basal and GC electrodes preferentially form H2O2 as the product through the two-electron pathway via inner and outer sphere mechanisms, respectively. The mechanism is significantly affected by the potential of maximal entropy, which determines the position of species in the solution within the inner or outer Helmholtz plane. The influence of alkali cations (Li+, Na+, K+, and Cs+) on the oxygen reduction reaction of these model carbon electrodes was investigated. Large cations, e.g., K+ and Cs+, showed influence on the reaction intermediates and thus on the electrodes’ selectivity. The present study provides important insights and contributions to the fundamental aspects of hydrogen peroxide production in acidic conditions and further advancements in the development of metal-free carbon-based catalysts.
碳电极上生成过氧化氢的电化学启示:电解质中缺陷、氧官能团和碱金属的影响
过氧化氢(H2O2)是一种环境友好型氧化剂,其产量到 2028 年将达到 570 万吨,市场规模到 2029 年将达到 40.4 亿美元。因此,了解氧气还原成 H2O2 的机理以及碳材料上的结构-活性关系,对于更环保地合成这种重要的化学物质具有重要意义。我们使用定向热解石墨(PG-edge 和 PG-basal)和玻璃碳(GC)作为模型电极,研究了碳缺陷、含氧官能团和碱金属的存在对酸性条件下产生 H2O2 的活性和选择性的影响。旋转环盘电极和电化学阻抗谱等电化学测量以及原位拉曼光谱电化学研究表明,PG-basal 和 GC 电极分别通过内球和外球机制,通过双电子途径优先形成 H2O2 作为产物。该机制受最大熵电位的影响很大,而最大熵电位决定了溶液中物种在内亥姆霍兹平面或外亥姆霍兹平面的位置。研究了碱阳离子(Li+、Na+、K+ 和 Cs+)对这些模型碳电极氧还原反应的影响。大阳离子(如 K+ 和 Cs+)对反应中间产物有影响,因此对电极的选择性也有影响。本研究对酸性条件下生产过氧化氢的基本方面以及无金属碳基催化剂的进一步发展提供了重要的见解和贡献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


