Magnesium oxide-supported potassium hydride as a transition-metal-free catalyst for the selective hydrogenation of alkynes
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Catalytic hydrogenation of alkynes to alkenes is pivotal in both petrochemical and fine chemical industries. Its implementation relies on the use of transition metals, especially those precious Pd-based catalysts. In this work, we utilize a melt infiltration method to prepare magnesium oxide-supported potassium hydride (KH/MgO), which is illustrated as a transition-metal-free catalyst for selective hydrogenation of alkynes. H2-D2 exchange experiment proves that dihydrogen activation and dissociation on KH/MgO is plausible at room temperature and ambient pressure. KH/MgO catalyzes hydrogenation of diphenylacetylene (DPA) already at 40 ℃, implying its high intrinsic activity at very mild conditions. Under an optimized condition of 80°C, 2 bar H2, and 40 h, 75 % conversion of DPA is achieved, affording 82 % selectivity to stilbenes. Mechanistic study suggests that surface hydride on KH/MgO plays an important role toward dihydrogen activation, and involved in the hydrogenation step to form stilbenes. This work shows the promise for using light metal hydrides consisting of earth-abundant elements as alternative hydrogenation catalysts.
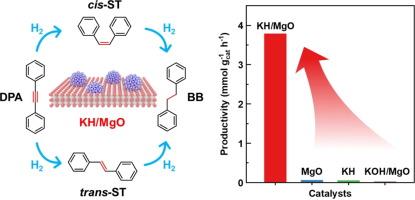
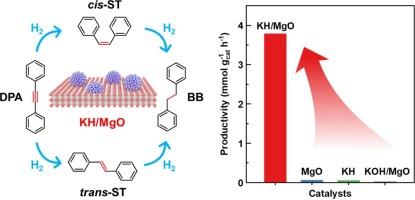
氧化镁支撑的氢化钾作为炔烃选择性氢化的无过渡金属催化剂
将炔烃催化氢化为烯烃在石化和精细化工行业都至关重要。其实施依赖于过渡金属的使用,尤其是那些珍贵的钯基催化剂。在这项工作中,我们利用熔融渗透法制备了氧化镁支撑的氢化钾(KH/MgO),并将其作为炔烃选择性氢化的无过渡金属催化剂加以说明。H2-D2 交换实验证明,在室温和环境压力下,KH/MgO 上的二氢活化和解离是可行的。KH/MgO 在 40 ℃ 时就能催化二苯基乙炔(DPA)的加氢反应,这表明它在非常温和的条件下具有很高的内在活性。在 80℃、2 巴 H2 和 40 小时的优化条件下,DPA 的转化率达到 75%,对二苯乙烯的选择性达到 82%。机理研究表明,KH/MgO 表面的氢化物在二氢活化过程中发挥了重要作用,并参与了氢化步骤以生成二苯乙烯。这项工作表明,使用由地球富集元素组成的轻金属氢化物作为替代氢化催化剂大有可为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
文献相关原料
公司名称
产品信息
阿拉丁
tridecane
阿拉丁
4-octyne
阿拉丁
1-octyne
阿拉丁
1-chloro-4-ethynylbenzene
阿拉丁
4-ethynyltoluene
阿拉丁
phenylacetylene
阿拉丁
bibenzyl
阿拉丁
trans-stilbene
阿拉丁
cis-stilbene
阿拉丁
diphenylacetylene
阿拉丁
oxalic acid dihydrate
阿拉丁
magnesium acetate tetrahydrate
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


