Unravelling the mechanistic ‘Black Box’ of heterogeneous condensation reactions catalyzed by aminosilicas
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
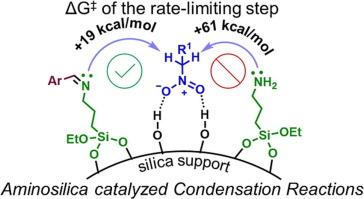
Primary amino-functionalized silicas (H2N-SiO2) are well known acid-base cooperative catalysts for many organic transformations, including carbon–carbon (C–C) bond forming condensation reactions, and much attention has been devoted to the elucidation of their action mode. However, to our surprise, the mechanism of Henry reactions and Knoevenagel condensations catalyzed by H2N-SiO2 is still paradoxical, and the identity of the actual base species, transition states, reactivity, and product selectivity, remain as debatable topics of discussion. Herein we propose a brand-new reaction mechanism for H2N-SiO2-catalyzed Henry reactions that overcomes all prior inconsistencies. With the aid of Hammett analysis and density functional theory (DFT) calculations, we have effectively identified several critical transition states and are able to explain reactivity and product selectivity. This study revealed that H2N-SiO2 catalyzed Henry reactions of aldehydes and nitro compounds follow the imine mechanism to afford olefin adducts as only possible products. In addition, we utilized our findings to comprehend the mechanism of Knoevenagel condensation, a comparable reaction, dispelling a more than two-decade old misconception regarding the nature of the active base involved.
揭开氨基硅酸催化的异质缩合反应的机理 "黑匣子
原氨基官能团二氧化硅(H2N-SiO2)是众所周知的酸碱协同催化剂,可催化许多有机转化反应,包括碳-碳(C-C)键形成的缩合反应,人们对其作用模式的阐明也给予了极大的关注。然而,令我们惊讶的是,H2N-SiO2 催化的 Henry 反应和 Knoevenagel 缩合反应的机理仍然是自相矛盾的,实际碱种类的身份、过渡态、反应活性和产物选择性仍然是值得讨论的话题。在此,我们为 H2N-SiO2 催化的亨利反应提出了一种全新的反应机理,克服了之前所有的不一致之处。借助 Hammett 分析和密度泛函理论 (DFT) 计算,我们有效地确定了几个临界过渡态,并解释了反应性和产物选择性。这项研究发现,H2N-SiO2 催化的醛和硝基化合物的亨利反应遵循亚胺机理,生成的烯烃加合物是唯一可能的产物。此外,我们还利用我们的研究成果理解了类似反应 Knoevenagel 缩合的机理,从而消除了二十多年前关于活性碱性质的误解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


