Diethyl Phosphate-Catalyzed One-Pot Synthesis of 2,3-Dihydroquinolin-4(1H)-ones from 2-Aminoacetophenones and Benzaldehydes
IF 1.9
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A diethyl phosphate (DEP)-catalyzed one-pot synthesis of 2,3-dihydroquinolin-4(1H)-ones in DMF was achieved. Control experiments led to a plausible mechanism involving an intermolecular aldol condensation of 2-aminoacetophenone and aldehyde, followed by a hydrogen bond-driven cyclization. In this process, a series of 2,3-dihydroquinolin-4(1H)-ones were obtained in good yields (48–84%). This strategy features transition metal-free and short reaction times.
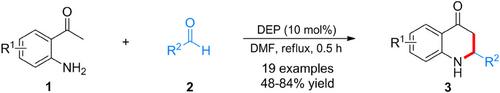
磷酸二乙酯催化的 2,3-二氢喹啉-4(1H)-酮与 2-氨基苯乙酮和苯甲醛的一锅合成反应
在 DMF 中实现了磷酸二乙酯 (DEP) 催化的 2,3-二氢喹啉-4(1H)-酮的一锅合成。对照实验得出了一个合理的机理,即 2-氨基苯乙酮和醛发生分子间醛醇缩合,然后氢键驱动环化。在此过程中,获得了一系列 2,3-二氢喹啉-4(1H)-酮,收率良好(48-84%)。这种策略的特点是不含过渡金属,反应时间短。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ChemistrySelect
Chemistry-General Chemistry
CiteScore
3.30
自引率
4.80%
发文量
1809
审稿时长
1.6 months
期刊介绍:
ChemistrySelect is the latest journal from ChemPubSoc Europe and Wiley-VCH. It offers researchers a quality society-owned journal in which to publish their work in all areas of chemistry. Manuscripts are evaluated by active researchers to ensure they add meaningfully to the scientific literature, and those accepted are processed quickly to ensure rapid online publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


