Cobalt-Promoted Para C─H Amination of 3-Acetyl Substituted Nitroarenes with Arylhydrazines
IF 1.9
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
We report a method to furnish substituted 1H-indazoles via a coupling of 3-acetyl substituted nitroarenes and arylhydrazines. Reactions progressed in the presence of cobalt(II) acetylacetonate catalyst, TEMPO oxidant, and Cs2CO3 base. Functional groups including bromo, chloro, nitro, and trifluoromethoxy groups were compatible with reaction conditions. The proposed mechanism was based on a nucleophilic substitution of hydrogen para to nitro group. Our method feature a convenient pathway to directly obtain substituted 1H-indazoles which avoid the use of pre-functionalized starting materials. Indazole could play a role as the directing group for a palladium-catalyzed C(sp2)─H arylation with iodobenzene.
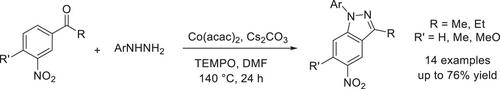
钴促进的 3-乙酰基取代硝基烯烃与芳基肼的 Para C─H Amination 反应
我们报告了一种通过 3-乙酰基取代的硝基烯烃和芳基肼偶联生成取代的 1H-吲唑的方法。反应在乙酰丙酮酸钴(II)催化剂、TEMPO 氧化剂和 Cs2CO3 碱存在下进行。包括溴、氯、硝基和三氟甲氧基在内的官能团与反应条件相容。提出的机理基于对位氢对硝基的亲核取代。我们的方法为直接获得取代的 1H-indazoles 提供了一条便捷的途径,避免了使用预官能化的起始材料。吲唑可以作为钯催化 C(sp2)-H 芳基化碘苯的指导基团。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ChemistrySelect
Chemistry-General Chemistry
CiteScore
3.30
自引率
4.80%
发文量
1809
审稿时长
1.6 months
期刊介绍:
ChemistrySelect is the latest journal from ChemPubSoc Europe and Wiley-VCH. It offers researchers a quality society-owned journal in which to publish their work in all areas of chemistry. Manuscripts are evaluated by active researchers to ensure they add meaningfully to the scientific literature, and those accepted are processed quickly to ensure rapid online publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


