Diastereoselective Access to Ester-Substituted cis-Phenanthridinones via Hydrogen Atom Transfer-Involved 1,7-Enyne Bicyclization
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-10-29
DOI:10.1021/acs.joc.4c0193710.1021/acs.joc.4c01937
引用次数: 0
Abstract
An organophotoredox-catalyzed 1,7-enyne bicyclization for the cis-diastereoselective synthesis of ester-substituted phenanthridinones has been achieved through radical cascade cyclization involving 1,6-hydrogen atom transfer. This transition-metal- and oxidant-free one-pot protocol generates three distinct C–C bonds and two quaternary carbon centers.
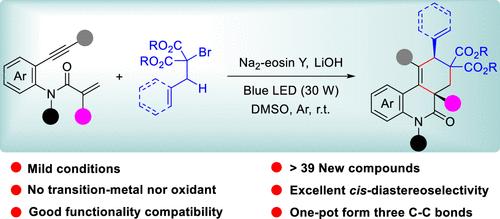
通过氢原子转移参与的 1,7-乙炔双环化非对映选择性获得酯取代的顺式菲啶酮
通过涉及 1,6 氢原子转移的自由基级联环化反应,实现了有机光oredox 催化的 1,7-enyne 双环化反应,从而顺式-非对映选择性地合成了酯取代的菲啶酮类化合物。这种不含过渡金属和氧化剂的一锅法生成了三个不同的 C-C 键和两个季碳中心。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


