Palladium-Catalyzed Synthesis of Substituted Phenanthrenes via a C–H Annulation of 2-Biaryl Triflates with Alkynes
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-10-28
DOI:10.1021/acs.joc.4c0136910.1021/acs.joc.4c01369
引用次数: 0
Abstract
A new palladium-catalyzed efficient method for the synthesis of substituted 9,10-phenanthrenes from 2-biaryl triflates with alkynes has been developed. This method provides a great opportunity to prepare various symmetrical and unsymmetrical phenanthrene derivatives in good yields. This reaction proceeds via C–OTf bond cleavage and alkyne insertion followed by C–H annulation.
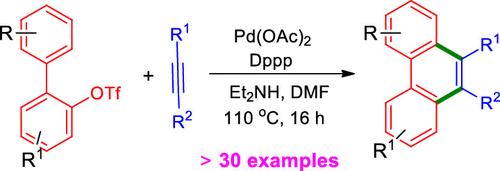
通过 2-Biaryl Triflates 与炔烃的 C-H 嵌合反应在钯催化下合成取代菲
我们开发了一种新的钯催化高效方法,用于从 2-联三酸酯与炔烃合成取代的 9,10-菲。该方法为制备各种对称和不对称菲衍生物提供了良好的产率。该反应通过 C-OTf 键裂解和炔烃插入后的 C-H 环化进行。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


