Regio-, Enantio-Controlled Cascade Oxa-Michael Addition of Hydroxyl Amine: Synthesis of Dihydro-1,2-Oxazines
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-11-07
DOI:10.1021/acs.joc.4c0208010.1021/acs.joc.4c02080
引用次数: 0
Abstract
Herein, we disclose a cinchona-alkaloid-based squaramide-catalyzed intermolecular oxa-Michael cascade addition cyclization of N-protected hydroxyl amine to formyl-tethered Michael acceptors toward the synthesis of enantioenriched 1,2-oxazine scaffolds. Generally, good yield (up to 78%), good to excellent enantiomeric ratio (up to 98:2 er), and excellent regioselectivity (>19:1) were observed. Furthermore, the scalability of this methodology and a successful demonstration of postsynthetic transformations have been accomplished.
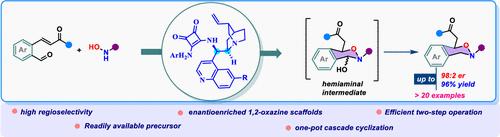
对映控制的羟胺级联 Oxa-Michael 加成:二氢-1,2-噁嗪的合成
在此,我们公开了一种基于金鸡纳类生物碱的方酰胺催化分子间氧杂迈克尔级联加成环化反应,将 N-保护羟胺与甲酰基系迈克尔受体环化,从而合成对映体丰富的 1,2-噁嗪支架。一般来说,该方法具有良好的产率(高达 78%)、良好到极佳的对映体比例(高达 98:2)和极佳的区域选择性(19:1)。此外,该方法的可扩展性和合成后转化的成功示范也已完成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


