Light-Induced Difunctionalization of Alkenes with Polyhaloalkanes and Quinoxalin-2(1H)-ones
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-11-01
DOI:10.1021/acs.joc.4c0211910.1021/acs.joc.4c02119
引用次数: 0
Abstract
Herein, we report a metal-free light-induced three-component reaction for the synthesis of polychloroalkyl-substituted quinoxalin-2(1H)-ones using commercially available alkenes, polyhalo alkanes, and quinoxalin-2(1H)-ones. Preliminary mechanistic studies suggested the generation of radical intermediates via an EDA-complex, single electron transfer, or halogen atom transfer pathway. Under mild reaction conditions, various alkenes and quinoxalin-2(1H)-ones containing different functional groups are compatible, providing the corresponding polychloroalkyl-substituted quinoxalin-2(1H)-ones in moderate to good yields.
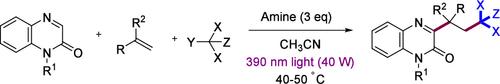
光诱导烯与多卤烷烃和喹喔啉-2(1H)-酮的双官能化作用
在此,我们报告了一种利用市售烯、多卤烷烃和喹喔啉-2(1H)-酮合成多氯烷基取代的喹喔啉-2(1H)-酮的无金属光诱导三组分反应。初步机理研究表明,自由基中间体是通过 EDA 复合物、单电子转移或卤原子转移途径生成的。在温和的反应条件下,各种烯类和含有不同官能团的喹喔啉-2(1H)-酮均能兼容,并以中等至良好的产率提供相应的多氯烷基取代的喹喔啉-2(1H)-酮。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


