Phosphine-Catalyzed [4 + 1] Annulation of β’-Acetoxy Allenoate with α-Alkylidene Succinimides: Access to Functionalized Spirosuccinimide Derivatives
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-10-31
DOI:10.1021/acs.joc.4c0201410.1021/acs.joc.4c02014
引用次数: 0
Abstract
A phosphine-catalyzed [4 + 1] annulation of β’-acetoxy allenoate with α-alkylidene succinimides is described. This method demonstrates the nucleophilic dialkylation and cyclization of α-alkylidene succinimides, resulting in the formation of functionalized spirosuccinimide derivatives. The reaction exhibits a wide substrate scope and yields ranging from moderate to excellent under the optimized conditions. In addition, the biological evaluation indicates that the cycloadduct 3u presents satisfied inhibitory activities for three human cancer cell lines (HCT116, A549, and HepG2).
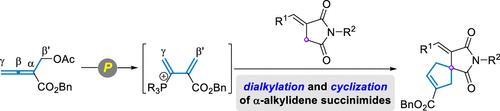
磷化氢催化 β'-Acetoxy Allenoate 与 α-Alkylidene Succinimides 的 [4 + 1] 嵌合反应:获得功能化的螺琥珀酰亚胺衍生物
介绍了一种磷化氢催化的 β'-acetoxy allenoate 与 α- 亚烷基琥珀酰亚胺的 [4 + 1] 环化反应。该方法展示了 α-亚烷基琥珀酰亚胺的亲核二烷基化和环化,从而形成官能化的螺琥珀酰亚胺衍生物。在优化的条件下,该反应的底物范围很广,产率从中等到极好不等。此外,生物学评估表明,环加合物 3u 对三种人类癌细胞株(HCT116、A549 和 HepG2)具有满意的抑制活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


