Direct Synthesis of Benzothiazoles and Benzoxazoles from Carboxylic Acids Utilizing (o-CF3PhO)3P as a Coupling Reagent
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-10-24
DOI:10.1021/acs.joc.4c0180710.1021/acs.joc.4c01807
引用次数: 0
Abstract
A general and efficient method for the direct synthesis of benzothiazoles and benzoxazoles from carboxylic acids with 2-aminobenzenethiols or 2-aminophenols using (o-CF3PhO)3P as a simple coupling reagent has been developed. Diverse benzothiazoles and benzoxazoles were synthesized in moderate to excellent yields. And the gram-scale preparation of benzothiazole and benzoxazole also proceeded smoothly under the mild conditions. Moreover, a plausible reaction mechanism was discussed, with (o-CF3PhO)3P and its hydrolysis product (o-CF3PhO)2P(O)H contributing to the formation of the target products as an amide synthesis coupling agent and a cyclization reaction promoter, respectively.
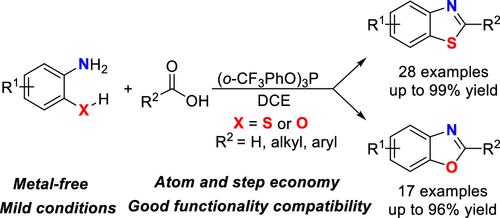
利用 (o-CF3PhO)3P 作为偶联试剂从羧酸直接合成苯并噻唑和苯并恶唑
以(o-CF3PhO)3P 作为简单的偶联试剂,开发了一种从羧酸与 2-氨基苯硫酚或 2-氨基苯酚直接合成苯并噻唑和苯并恶唑的通用而高效的方法。合成了多种苯并噻唑和苯并恶唑,收率从中等到极好。在温和的条件下,以克为单位制备苯并噻唑和苯并恶唑也进展顺利。此外,还讨论了一种合理的反应机理,即(o-CF3PhO)3P及其水解产物(o-CF3PhO)2P(O)H分别作为酰胺合成偶联剂和环化反应促进剂促进了目标产物的形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


