Palladium-Catalyzed Electrochemical Iodination of 1-Arylpyridine N-Oxides
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-02-27
DOI:10.1021/acs.joc.3c0260110.1021/acs.joc.3c02601
引用次数: 0
Abstract
The palladium-catalyzed C–H iodination of 1-arylpyridine N-oxides proceeded under electrochemical oxidation conditions using I2 as an iodine source. The reaction of isoquinoline N-oxides possessing various para- or meta-substituted aryl groups at the 1-position proceeded to give the corresponding iodination products. Electron-donating groups on the aryl group facilitated the reaction to give relatively high yields of the product. The reaction was also found to be applicable to 2-aryl-3-picoline N-oxides.
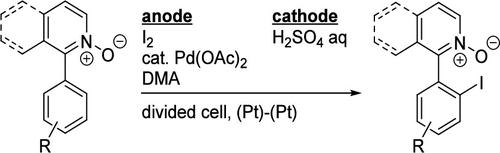
钯催化的 1-芳基吡啶 N-氧化物电化学碘化反应
在电化学氧化条件下,以 I2 为碘源,钯催化了 1-芳基吡啶 N-氧化物的 C-H 碘化反应。1 位上具有各种对位或偏取代芳基的异喹啉 N-氧化物在反应过程中生成了相应的碘化产物。芳基上的电子供能基团促进了反应的进行,从而得到相对较高产率的产物。该反应还适用于 2-芳基-3-甲基吡啶 N-氧化物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


