Aryliodonium Salt-Induced Regioselective Access to meta-Substituted Anilines by Arylation of Azoles
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-02-22
DOI:10.1021/acs.joc.3c0241710.1021/acs.joc.3c02417
引用次数: 0
Abstract
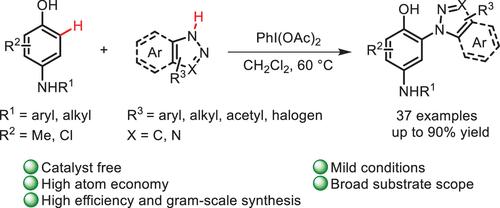
A highly efficient aryliodonium salt-induced regioselective access to meta-substituted anilines by arylation of azoles has been developed under catalyst-free conditions. This efficient transformation provides a facile and scalable approach to a wide range of biologically active N-arylazoles with moderate to high yields. According to the control experiments, two plausible pathways, including a Michael pathway and a free radical coupling pathway, for the reaction were proposed.
芳基碘鎓盐通过芳基化偶氮类化合物诱导对元取代苯胺的区域选择性获取
在无催化剂条件下,通过芳基化偶氮唑,开发出了一种由芳基碘鎓盐诱导的高效区域选择性方法。这种高效的转化提供了一种简便、可扩展的方法,可以中等至高产率获得多种具有生物活性的 N-芳基唑。根据对照实验,提出了该反应的两种可行途径,包括迈克尔途径和自由基偶联途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


