Light-assisted functionalization of aryl radicals towards metal-free cross-coupling
IF 38.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The many synthetic possibilities that arise when using radical intermediates, in place of their polar counterparts, make contemporary radical chemistry research an exhilarating field. The introduction of photocatalysis has helped tame aryl radicals, leading to a resurgence of interest in their chemistry, and an expansion of viable coupling partners and attainable transformations. These methods are more selective and safer than classical approaches, and they utilize new radical precursors. Given the importance of sustainability in current organic synthesis and our interest in light-assisted metal-free transformations, this Review focuses on recent advances in the use of aryl radicals in photoinduced cross-couplings that do not rely on metals for the crucial bond-forming event, and it is structured according to the key step that the aryl radicals engage in. Our improved understanding of how to tame aryl radicals means they are now used in many transformations to access high-value products. This Review provides a summary of contemporary approaches towards the photogeneration of aryl radicals and their use in metal-free cross-coupling reactions.
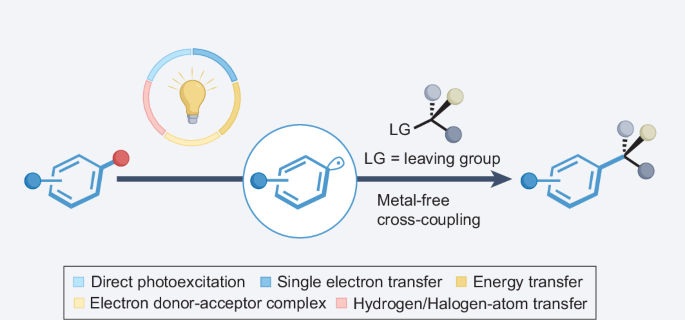

光助芳基自由基官能化,实现无金属交叉耦合。
当使用自由基中间体代替极性对应物时,会产生许多合成可能性,这使得当代自由基化学研究成为一个令人振奋的领域。光催化技术的引入帮助驯服了芳基自由基,使人们对它们的化学性质重新产生了兴趣,并扩大了可行的偶联伙伴和可实现的转化。与传统方法相比,这些方法的选择性更强、更安全,而且利用了新的自由基前体。鉴于可持续发展在当前有机合成中的重要性以及我们对光辅助无金属转化的兴趣,本综述将重点介绍芳基自由基在光诱导交叉耦合中的最新应用进展,这些方法在关键的成键过程中不依赖金属。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature reviews. Chemistry
Chemical Engineering-General Chemical Engineering
CiteScore
52.80
自引率
0.80%
发文量
88
期刊介绍:
Nature Reviews Chemistry is an online-only journal that publishes Reviews, Perspectives, and Comments on various disciplines within chemistry. The Reviews aim to offer balanced and objective analyses of selected topics, providing clear descriptions of relevant scientific literature. The content is designed to be accessible to recent graduates in any chemistry-related discipline while also offering insights for principal investigators and industry-based research scientists. Additionally, Reviews should provide the authors' perspectives on future directions and opinions regarding the major challenges faced by researchers in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


