Prenatal choline supplementation enhances metabolic outcomes with differential impact on DNA methylation in Wistar rat offspring and dams
IF 4.8
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Choline is an essential nutrient required for proper functioning of organs and serves as a methyl donor. In liver where choline metabolism primarily occurs, glucose homeostasis is regulated through insulin receptor substrates (IRS) 1 and 2. The objective of this research was to determine the role of prenatal choline as a modulator of metabolic health and DNA methylation in liver of offspring and dams. Pregnant Wistar rat dams were fed an AIN-93G diet and received drinking water either with supplemented 0.25% choline (w/w) as choline bitartrate or untreated control. All offspring were weaned to a high-fat diet for 12 weeks. Prenatal choline supplementation led to higher insulin sensitivity in female offspring at weaning as well as lower body weight and food intake and higher insulin sensitivity in female and male adult offspring compared to offspring from untreated dams. Higher hepatic betaine concentrations were observed in dams and female offspring of choline-supplemented dams at weaning and higher glycerophosphocholine in female and male offspring at postweaning compared to the untreated control, suggestive of sustaining different choline pathways. Hepatic gene expression of Irs2 was higher in dams at weaning and female offspring at weaning and postweaning, whereas Irs1 was lower in male offspring at postweaning. Gene-specific DNA methylation of Irs2 was lower in female offspring at postweaning and Irs1 methylation was higher in male offspring at postweaning that exhibited an inverse relationship between methylation and gene expression. In conclusion, prenatal choline supplementation contributes to improved parameters of insulin signaling but these effects varied across time and offspring sex.
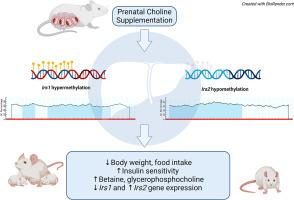
产前补充胆碱可提高 Wistar 大鼠后代和母鼠的代谢结果,并对 DNA 甲基化产生不同影响。
胆碱是器官正常运作所必需的营养物质,也是甲基供体。在主要进行胆碱代谢的肝脏中,葡萄糖稳态是通过胰岛素受体底物(IRS)1 和 2 调节的。在此,我们确定了产前胆碱作为子代和母鼠肝脏代谢健康和 DNA 甲基化调节剂的作用。怀孕的 Wistar 大鼠母体喂食 AIN-93G 食物,并在饮用水中添加 0.25% 的胆碱(重量比),即酒石酸胆碱或未经处理的对照品。所有后代断奶后均以高脂肪饮食喂养 12 周。与未添加胆碱的母鼠的后代相比,产前添加胆碱的母鼠的后代断奶时胰岛素敏感性更高,成年雌性和雄性后代的体重和食物摄入量更低,胰岛素敏感性更高。与未添加胆碱的对照组相比,添加胆碱的母鼠和雌性后代在断奶时的肝脏甜菜碱浓度较高,断奶后雌性和雄性后代的甘油磷酸胆碱浓度较高,这表明胆碱的作用途径不同。Irs2在断奶母鼠和断奶及断奶后雌性后代中的肝脏基因表达量较高,而Irs1在断奶后雄性后代中的表达量较低。断奶后雌性后代中 Irs2 的基因特异性 DNA 甲基化程度较低,断奶后雄性后代中 Irs1 的甲基化程度较高,甲基化与基因表达之间呈反比关系。总之,产前补充胆碱有助于改善胰岛素信号转导的参数,但这些影响因时间和后代性别而异。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Nutritional Biochemistry
医学-生化与分子生物学
CiteScore
9.50
自引率
3.60%
发文量
237
审稿时长
68 days
期刊介绍:
Devoted to advancements in nutritional sciences, The Journal of Nutritional Biochemistry presents experimental nutrition research as it relates to: biochemistry, molecular biology, toxicology, or physiology.
Rigorous reviews by an international editorial board of distinguished scientists ensure publication of the most current and key research being conducted in nutrition at the cellular, animal and human level. In addition to its monthly features of critical reviews and research articles, The Journal of Nutritional Biochemistry also periodically publishes emerging issues, experimental methods, and other types of articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


