Homogeneity analysis of medicine tablets by laser induced breakdown spectroscopy combined with multivariate methods
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-11-14
DOI:10.1016/j.ejpb.2024.114579
引用次数: 0
Abstract
Pharmaceutical tablets need to have a homogenous chemical structure, especially in cases where the patient may divide the tablet in half prior to consumption. This work aims to demonstrate the viability of using laser induced breakdown spectroscopy (LIBS) for analyzing the homogeneity and determining the chemical composition of losartan potassium tablets. This was accomplished by obtaining the spectra of 10 tablet points in 30 successive laser pulses, which revealed four main peaks (C, H, N, and O) as well as a high concentration of calcium and potassium in the core tablets and titanium in the coating—all of which are excellent analytical objectives for LIBS. It is possible to say that the generated plasma meets the minimum requirement for local thermodynamic equilibrium because the physical parameters of the plasma, including temperature (T) and electronic density (Ne), were calculated throughout the Boltzmann plot and Stark broadened line, respectively, and the McWhirter criterion was met. In addition, T and Ne changes have been used for homogeneity analysis. Different peak comparisons cannot provide us with further data because the major structural components are similar, making it challenging to differentiate between them. So relative standard deviation (RSD) and principal component analysis (PCA) were used to comprise the whole spectra, which showed that the homogeneity of the tablet’s core is better than that of the coating and is acceptable.
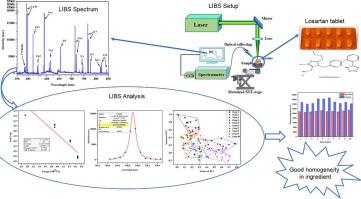
用激光诱导击穿光谱法结合多元方法分析药片的均匀性。
药片需要具有均匀的化学结构,尤其是在患者服用前可能会将药片分成两半的情况下。这项研究旨在证明使用激光诱导击穿光谱(LIBS)分析洛沙坦钾片的均匀性和确定其化学成分的可行性。通过连续 30 个激光脉冲获得 10 个片剂点的光谱,结果显示出四个主峰(C、H、N 和 O),以及片芯中高浓度的钙和钾和包衣中的钛--所有这些都是 LIBS 的绝佳分析目标。可以说,生成的等离子体符合局部热力学平衡的最低要求,因为等离子体的物理参数,包括温度(T)和电子密度(Ne),分别是通过整个波尔兹曼图和斯塔克展宽线计算出来的,符合麦克维尔特标准。此外,T 和 Ne 的变化也被用于均匀性分析。不同峰值的比较不能为我们提供进一步的数据,因为主要的结构成分都很相似,要区分它们很困难。因此,我们使用相对标准偏差(RSD)和主成分分析(PCA)来组成整个光谱,结果表明片剂药芯的均匀性优于包衣,是可以接受的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


