The establishment of the anther somatic niche with single-cell sequencing
IF 2.5
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
Abstract
The anther is the developmental housing of pollen and therefore the male gametes of flowering plants. The meiotic cells from which pollen are derived must differentiate de novo from somatic anther cells and synchronously develop with the rest of the anther. Anthropogenic control over another development has become crucial for global agriculture so as to maintain inbred lines and generate hybrid seeds of many crops. Understanding the genes that underlie the proper differentiation, developmental landmarks, and functions of each anther cell type is thus fundamental to both basic and applied plant sciences. We investigated the development of the somatic niche of the maize (Zea mays) anther using single-cell RNA-seq (scRNA-seq). Extensive background knowledge on the birth then pace and pattern of cell division of the maize anther cell types and published examples of cell-type gene expression from in situ hybridization allowed us to identify the primary cell types within the anther lobe, as well as the connective cells between the four lobes. We established the developmental trajectories of somatic cell types from pre-meiosis to post-meiosis, identified putative marker genes for the somatic cell types that previously lacked any known specific functions, and addressed the possibility that tapetal cells sequentially differentiate. This comprehensive scRNA-seq dataset of the somatic niche of the maize anther will serve as a baseline for future analyses investigating male-sterile genotypes and the impact of environmental conditions on male fertility in flowering plants.
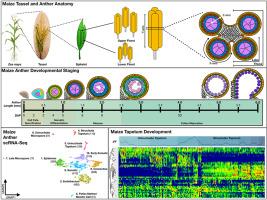
利用单细胞测序建立花药体细胞龛。
花药是花粉的发育场所,因此也是开花植物雄配子的发育场所。产生花粉的减数分裂细胞必须从体细胞中重新分化出来,并与花药的其他部分同步发育。人类对花药发育的控制已成为全球农业的关键,以便保持近交系和产生许多作物的杂交种子。因此,了解每种花药细胞类型的适当分化、发育标志和功能的基础基因对于植物基础科学和应用科学都至关重要。我们利用单细胞 RNA-seq(scRNA-seq)研究了玉米(Zea mays)花药体细胞龛的发育。有关玉米花药细胞类型的诞生、分裂速度和模式的广泛背景知识,以及已发表的原位杂交细胞类型基因表达实例,使我们能够确定花药裂片内的主要细胞类型,以及四个裂片之间的连接细胞。我们确定了体细胞类型从减数分裂前到减数分裂后的发育轨迹,鉴定了以前缺乏任何已知特定功能的体细胞类型的假定标记基因,并探讨了绦虫细胞顺序分化的可能性。这一全面的玉米花药体细胞生态位 scRNA-seq 数据集将作为未来研究雄性不育基因型和环境条件对开花植物雄性生殖力影响的分析基线。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


