Extension of impurity profiling on eltrombopag olamine to in-silico predictions: An effort to exploit correlated forced degradation products and known drug-related substances in drug discovery
IF 2.8
3区 医学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
The recent pandemic has highlighted the impact of diseases on global health and the economy. The rapid discovery of new hit molecules remains a tough challenge. Pharmaceutical impurity profiling can be linked to drug discovery through the identification of new hits from compounds identified during the analytical profiling. The present study demonstrates this linkage through the extension of the impurity (forced degradation) profiling of eltrombopag (ELT) olamine, a thrombopoietin (TPO) receptor agonist. The drug was exposed to standard degradation and the degradation products were primarily resolved and identified by UPLC-ESI-MS. This led to the identification of five forced degradation products (FDP). Thirty-three other known related substances (RS) of ELT, identified in the literature, were also considered. Molecular similarity checks were performed using Tanimoto/Jaccard's similarity searches. A set of structurally and topologically similar molecules, including ELT and 15 RS, was established and subjected to in-silico toxicity-, absorption-, distribution-, metabolism-, and elimination (ADME) predictions. The RS, predicted with similar or lower toxicity than ELT and a comparable ADME profile, were subjected to molecular docking to trace changes in TPO receptor affinity. The results indicated that five RS had a high Jaccard’s similarity with ELT and higher or comparable docking scores. These compounds, along with few other impurities were predicted to have lower toxicity, better or comparable absorption, distribution, metabolism, and also a better excretion profile than ELT. This justifies their entry as potential novel TPO receptor agonists in drug discovery.
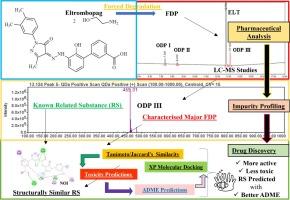
将艾曲波帕乙醇胺上的杂质分析扩展到实验室预测:在药物发现中利用相关强制降解产物和已知药物相关物质的努力。
最近的大流行突显了疾病对全球健康和经济的影响。快速发现新的热门分子仍然是一项严峻的挑战。药物杂质分析可以通过从分析过程中发现的化合物中识别出新的热门分子,从而与药物发现联系起来。本研究通过扩展对凝血酶原(TPO)受体激动剂艾曲波帕(ELT)橄榄胺的杂质(强制降解)分析,证明了这种联系。对药物进行标准降解,降解产物主要通过 UPLC-ESI-MS 进行分辨和鉴定。最终确定了五种强制降解产物(FDP)。此外,还考虑了文献中发现的其他 33 种已知的 ELT 相关物质 (RS)。使用 Tanimoto/Jaccard 相似性搜索进行了分子相似性检查。建立了一组结构和拓扑相似的分子(包括 ELT 和 15 种 RS),并对其进行了体内毒性、吸收、分布、代谢和消除(ADME)预测。预测出的 RS 具有与 ELT 相似或更低的毒性和可比的 ADME 特征,并对其进行了分子对接,以追踪 TPO 受体亲和力的变化。结果表明,有五种 RS 与 ELT 的 Jaccard 相似度较高,对接得分也较高或相当。据预测,与 ELT 相比,这些化合物以及少量其他杂质的毒性更低,吸收、分布、代谢和排泄情况更好或相当。这就证明它们有可能成为药物研发中的新型 TPO 受体激动剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chromatography B
医学-分析化学
CiteScore
5.60
自引率
3.30%
发文量
306
审稿时长
44 days
期刊介绍:
The Journal of Chromatography B publishes papers on developments in separation science relevant to biology and biomedical research including both fundamental advances and applications. Analytical techniques which may be considered include the various facets of chromatography, electrophoresis and related methods, affinity and immunoaffinity-based methodologies, hyphenated and other multi-dimensional techniques, and microanalytical approaches. The journal also considers articles reporting developments in sample preparation, detection techniques including mass spectrometry, and data handling and analysis.
Developments related to preparative separations for the isolation and purification of components of biological systems may be published, including chromatographic and electrophoretic methods, affinity separations, field flow fractionation and other preparative approaches.
Applications to the analysis of biological systems and samples will be considered when the analytical science contains a significant element of novelty, e.g. a new approach to the separation of a compound, novel combination of analytical techniques, or significantly improved analytical performance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


