Pulmonary delivery of dual-targeted nanoparticles improves tumor accumulation and cancer cell targeting by restricting macrophage interception in orthotopic lung tumors
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Despite the recognized potential of inhaled nanomedicines to enhance and sustain local drug concentrations for lung cancer treatment, the influence of macrophage uptake on targeted nanoparticle delivery to and within tumors remains unclear. Here, we developed three ligand-coated nanoparticles for pulmonary delivery in lung cancer therapy: phenylboronic acid-modified nanoparticles (PBA-NPs), PBA combined with folic acid (FA-PBA-NPs), and PBA with mannose (MAN-PBA-NPs). In vitro, MAN-PBA-NPs were preferentially internalized by macrophages, whereas FA-PBA-NPs exhibited superior uptake by cancer cells compared to macrophages. Following intratracheal instillation into mice with orthotopic Lewis lung carcinoma tumors, all three nanoparticles showed similar lung retention. However, MAN-PBA-NPs were more prone to interception by lung macrophages, which limited their accumulation in tumor tissues. In contrast, both PBA-NPs and FA-PBA-NPs achieved comparable high tumor accumulation (∼11.3% of the dose). Furthermore, FA-PBA-NPs were internalized by ∼30% of cancer cells, significantly more than the 10–18% seen with PBA-NPs or MAN-PBA-NPs. Additionally, FA-PBA-NPs loaded with icaritin effectively inhibited the Wnt/β-catenin pathway, resulting in superior anti-tumor efficacy through targeted cancer cell delivery. Overall, FA-PBA-NPs demonstrated advantageous competitive uptake kinetics by cancer cells compared to macrophages, enhancing tumor targeting and therapeutic outcomes.
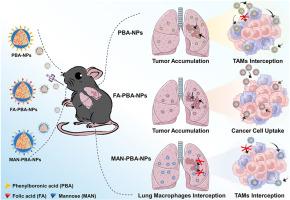
双靶向纳米粒子的肺部给药通过限制巨噬细胞对正位肺肿瘤的拦截,改善了肿瘤积累和癌细胞靶向性。
尽管吸入纳米药物在提高和维持肺癌治疗的局部药物浓度方面具有公认的潜力,但巨噬细胞摄取对纳米粒子靶向输送到肿瘤和肿瘤内的影响仍不清楚。在此,我们开发了三种配体包裹的纳米颗粒,用于肺癌治疗的肺部给药:苯硼酸修饰纳米颗粒(PBA-NPs)、苯硼酸与叶酸结合的纳米颗粒(FA-PBA-NPs)和苯硼酸与甘露糖结合的纳米颗粒(MAN-PBA-NPs)。在体外,MAN-PBA-NPs 优先被巨噬细胞内化,而 FA-PBA-NPs 与巨噬细胞相比更容易被癌细胞吸收。在对小鼠进行气管内灌注后,这三种纳米粒子都显示出相似的肺部滞留性。但是,MAN-PBA-NPs 更容易被肺巨噬细胞截获,从而限制了其在肿瘤组织中的积累。相比之下,PBA-NPs和FA-PBA-NPs的肿瘤蓄积量相当高(占剂量的11.3%)。此外,FA-PBA-NPs 被 30% 的癌细胞内化,明显高于 PBA-NPs 或 MAN-PBA-NPs 的 10-18%。此外,装载了icaritin的FA-PBA-NPs还能有效抑制Wnt/β-catenin通路,从而通过靶向癌细胞递送产生卓越的抗肿瘤效果。总之,与巨噬细胞相比,FA-PBA-NPs 在癌细胞的竞争性吸收动力学方面更具优势,从而提高了肿瘤靶向性和治疗效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


