Enhanced Long-Term Stability of Zinc-Air Batteries Using a Quaternized PVA-Chitosan Composite Separator with Thin-Layered MoS2
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
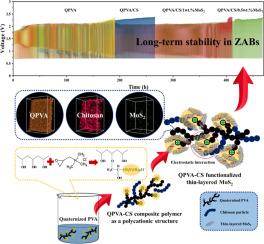
Due to their high energy density and cost-effectiveness, rechargeable zinc-air batteries (ZABs) are increasingly recognized for their potential as long-duration energy storage solutions. A crucial component for maximizing their efficiency is the membrane separator, which must exhibit high hydroxide-ion conductivity and long-term stability. This study introduces an innovative hybrid composite separator, created by embedding thin-layered molybdenum disulfide (MoS2), known for its intrinsic negative charge, into a polycationic quaternized PVA-chitosan (CS) matrix. Polyvinyl alcohol (PVA) is functionalized with quaternary ammonium (QA) groups before being combined with CS and MoS2, using a solvent blending technique. The separator's three-dimensional structure and morphology is analyzed via synchrotron radiation X-ray tomographic microscopy (SR-XTM). Results demonstrate that the ZAB equipped with a quaternized PVA/CS/0.5 wt.% MoS2 composite separator achieved a high conductivity of 87.3 mS cm-1 and exceptional stability, enduring over 465 cycles. This performance is attributed to the synergistic interaction between the quaternized PVA-CS matrix and the MoS2 surface, forming robust polymer complexes through electrostatic interactions. These findings suggest that the developed separators hold significant promise for advanced ZAB applications.
使用带有薄层 MoS2 的季铵化 PVA-壳聚糖复合分离器增强锌-空气电池的长期稳定性
可充电锌-空气电池(ZAB)具有高能量密度和成本效益,因此越来越多的人认识到其作为长时间能量存储解决方案的潜力。要最大限度地提高其效率,膜分离器是一个关键部件,它必须具有高氢氧离子传导性和长期稳定性。本研究介绍了一种创新的混合复合分离器,它是通过将薄层二硫化钼(MoS2)嵌入聚阳离子季铵化 PVA-壳聚糖(CS)基质而制成的,MoS2 以其固有的负电荷而著称。聚乙烯醇(PVA)在与 CS 和 MoS2 结合之前,先用季铵(QA)基团进行官能化,然后使用溶剂混合技术进行混合。分离器的三维结构和形态通过同步辐射 X 射线断层显微镜(SR-XTM)进行分析。结果表明,配备了季铵化 PVA/CS/0.5 wt.% MoS2 复合分离器的 ZAB 实现了 87.3 mS cm-1 的高电导率和卓越的稳定性,可经受 465 次循环。这一性能归功于季铵化 PVA-CS 基质与 MoS2 表面之间的协同作用,通过静电作用形成了稳健的聚合物复合物。这些研究结果表明,所开发的分离器在先进的 ZAB 应用中大有可为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


