Expedited Aminoglutarimide C–N Cross-Coupling Enabled by High-Throughput Experimentation
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A simple protocol for the Buchwald–Hartwig cross-coupling of (hetero)aryl halides with unprotected aminoglutarimide to afford diverse cereblon binding motifs is disclosed. The development of this C–N cross-coupling method was enabled by high-throughput combinatory screening of solvents, bases, temperatures, and ligands. Scope studies revealed generality across various heteroaryl and aryl halides with the reaction proceeding under mild conditions. In comparison, this method demonstrated strategic superiority over previously reported approaches, as evidenced by a significant decrease in step count from known syntheses in the patent literature.
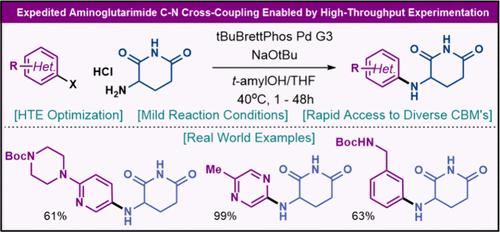
通过高通量实验实现快速氨基戊二酰亚胺 C-N 交叉偶联
本研究揭示了(杂)芳基卤化物与未受保护的氨基戊二酰亚胺进行 Buchwald-Hartwig 交叉偶联以获得多种脑龙结合基团的简单方案。通过对溶剂、碱、温度和配体进行高通量组合筛选,开发出了这种 C-N 交叉偶联方法。范围研究表明,该方法适用于各种杂芳基和芳基卤化物,反应在温和的条件下进行。与之前报道的方法相比,这种方法在战略上更具优势,这体现在与专利文献中已知的合成方法相比,步骤数显著减少。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


