Hydrothermal liquefaction of diverse plastics using water and seawater
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
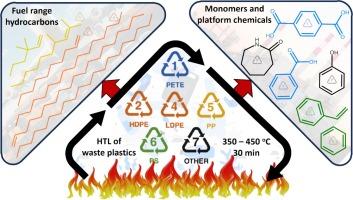
In this study, a variety of plastics are converted to their monomers, fuel range hydrocarbons and other platform chemicals using hydrothermal liquefaction (HTL) at sub-critical (350 °C), near-critical (400 °C) and super-critical (450 °C) temperatures. The effect of seawater as a medium on the HTL of plastics is also studied. Polymers like polycarbonate (PC), acrylonitrile–butadiene–styrene (ABS), polyamides (PA-6, PA-66), and polyoxymethylene (POM) liquefied effectively at 350 °C, while polyolefins like polyethylene (PE) and polypropylene (PP) required ≥ 400 °C. HTL of polystyrene (PS) resulted in the highest oil yield (93 wt%) and energy recovery from oil (93.5 %) at 450 °C. HTL of PC, PS and ABS led to the formation of phenols and benzene derivatives, while polyethylene terephthalate (PET) and PA-6 formed their monomers terephthalic acid and caprolactam, respectively. Crude oils from PP and PE contained fuel grade aliphatic hydrocarbons with carbon chain length ranging between C7 and C36. Highest calorific value was recorded for the oil from PP (46.3 MJ kg−1) at 400 °C. The use of seawater generally reduced the oil yields from all the plastics, except ABS, PET and PP. The production of benzoic acid from PET was enhanced, while the selectivity to caprolactam from PA-6 decreased when seawater was used, possibly due to the enhanced decarboxylation and aromatization reactions induced by the ions in the aqueous phase. In general, the crude oil from HTL of PC, PS, ABS and polyamides are suitable for sustainable production of specialty chemicals and monomers, while that from PE and PP are good candidates for sustainable transportation fuels.
利用水和海水对多种塑料进行水热液化
在这项研究中,在亚临界温度(350 °C)、近临界温度(400 °C)和超临界温度(450 °C)下,利用水热液化(HTL)将各种塑料转化为其单体、燃料范围内的碳氢化合物和其他平台化学品。还研究了海水作为介质对塑料 HTL 的影响。聚碳酸酯(PC)、丙烯腈-丁二烯-苯乙烯(ABS)、聚酰胺(PA-6、PA-66)和聚甲醛(POM)等聚合物在 350 ℃ 时有效液化,而聚乙烯(PE)和聚丙烯(PP)等聚烯烃则需要 ≥ 400 ℃。聚苯乙烯(PS)的热液化在 450 °C 时产油率最高(93 wt%),油的能量回收率也最高(93.5%)。PC、PS 和 ABS 的热液化产生了苯酚和苯衍生物,而聚对苯二甲酸乙二醇酯(PET)和 PA-6 则分别产生了对苯二甲酸和己内酰胺单体。聚丙烯和聚乙烯的原油含有燃料级脂肪烃,碳链长度在 C7 和 C36 之间。在 400 °C 时,PP 原油的热值最高(46.3 兆焦耳/千克-1)。除 ABS、PET 和 PP 外,使用海水普遍降低了所有塑料的产油量。使用海水时,PET 的苯甲酸产量增加,而 PA-6 的己内酰胺选择性降低,这可能是由于水相中的离子诱导脱羧和芳香化反应增强所致。总的来说,从 PC、PS、ABS 和聚酰胺的 HTL 中提取的原油适用于特种化学品和单体的可持续生产,而从 PE 和 PP 中提取的原油则是可持续运输燃料的良好候选材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


