Peroxynitrite-Free Nitric Oxide-Embedded Nanoparticles Maintain Nitric Oxide Homeostasis for Effective Revascularization of Myocardial Infarcts
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Revascularization is crucial for treating myocardial infarction (MI). Nitric oxide (NO), at an appropriate concentration, is recognized as an ideal and potent pro-angiogenic factor. However, the application of NO in the treatment of MI is limited. Improper NO supplementation is harmful to revascularization because NO is converted into harmful peroxynitrite (ONOO–) in MI tissues with high reactive oxygen species (ROS) levels. We overcome these obstacles by embedding biliverdin and NO into Prussian blue (PB) nanolattices to obtain an ONOO–-free NO-embedded nanomedicine (OFEN). Unlike previous NO donors, OFEN provides NO stably and spontaneously for a longer time (>7 days), which makes it possible to maintain a stable concentration of NO, suitable for angiogenesis, through dose optimization. More importantly, based on the synergy between PB and biliverdin, OFEN converts ROS into beneficial O2 and inhibits the production of ONOO– from the source. OFEN specifically targets MI tissues and achieves sustained and stable NO delivery at the MI site. OFEN effectively promotes revascularization in the MI tissue, significantly reduces myocardial death and fibrosis, and ultimately promotes the complete recovery of cardiac function. Our strategy provides a promising approach for the treatment of myocardial and other ischemic diseases.
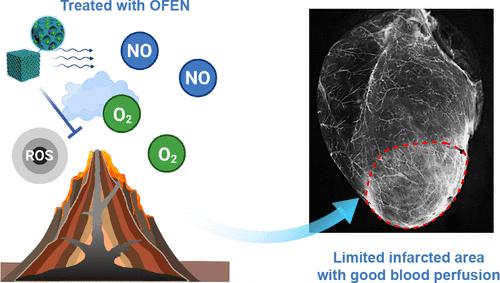
不含过亚硝酸盐的一氧化氮包埋纳米粒子可维持一氧化氮的平衡,从而实现心肌梗塞的有效血管再通
血管重建是治疗心肌梗塞(MI)的关键。适当浓度的一氧化氮(NO)被认为是一种理想而有效的促血管生成因子。然而,NO 在治疗心肌梗死中的应用还很有限。一氧化氮补充不当对血管再通有害,因为一氧化氮会在活性氧(ROS)水平较高的心肌梗死组织中转化为有害的过氧化亚硝酸盐(ONOO-)。我们克服了这些障碍,将胆绿素和氮氧化物嵌入普鲁士蓝(PB)纳米晶格,获得了不含ONOO的氮氧化物嵌入纳米药物(OFEN)。与以往的氮氧化物供体不同,OFEN能在更长的时间(7天)内稳定、自发地提供氮氧化物,这使得通过剂量优化来维持适合血管生成的稳定的氮氧化物浓度成为可能。更重要的是,基于 PB 和胆绿素的协同作用,OFEN 可将 ROS 转化为有益的 O2,并从源头抑制 ONOO- 的产生。OFEN 专门针对心肌梗死组织,在心肌梗死部位实现持续稳定的 NO 输送。OFEN 能有效促进心肌梗死组织的血管再通,显著减少心肌死亡和纤维化,并最终促进心脏功能的完全恢复。我们的策略为治疗心肌和其他缺血性疾病提供了一种前景广阔的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


