The single-molecule accessibility landscape of newly replicated mammalian chromatin
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
We present replication-aware single-molecule accessibility mapping (RASAM), a method to nondestructively measure replication status and protein-DNA interactions on chromatin genome-wide. Using RASAM, we uncover a genome-wide state of single-molecule “hyperaccessibility” post-replication that resolves over several hours. Combining RASAM with cellular models for rapid protein degradation, we demonstrate that histone chaperone CAF-1 reduces nascent chromatin accessibility by filling single-molecular “gaps” and generating closely spaced dinucleosomes on replicated DNA. At cis-regulatory elements, we observe unique modes by which nascent chromatin hyperaccessibility resolves: at CCCTC-binding factor (CTCF)-binding sites, CTCF and nucleosomes compete, reducing CTCF occupancy and motif accessibility post-replication; at active transcription start sites, high chromatin accessibility is maintained, implying rapid re-establishment of nucleosome-free regions. Our study introduces a new paradigm for studying replicated chromatin fiber organization. More broadly, we uncover a unique organization of newly replicated chromatin that must be reset by active processes, providing a substrate for epigenetic reprogramming.
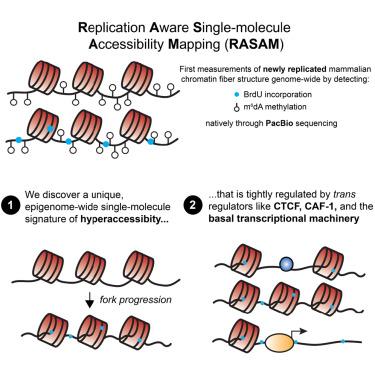
新复制的哺乳动物染色质的单分子可及性图谱
我们介绍了复制感知单分子可及性图谱(RASAM),这是一种无损测量全基因组染色质复制状态和蛋白质-DNA相互作用的方法。利用 RASAM,我们发现了一种复制后的全基因组单分子 "过度可及性 "状态,这种状态会在数小时内消失。结合 RASAM 和蛋白质快速降解的细胞模型,我们证明组蛋白伴侣 CAF-1 通过填补单分子 "间隙 "和在复制 DNA 上生成紧密间隔的二核体,降低了新生染色质的可及性。在顺式调控元件上,我们观察到了解决新生染色质过度可及性的独特模式:在 CCCTC 结合因子(CTCF)结合位点上,CTCF 与核小体发生竞争,从而降低了复制后 CTCF 的占据率和图案的可及性;在活跃的转录起始位点上,染色质的高可及性得以维持,这意味着无核小体区域的快速重建。我们的研究为研究复制染色质纤维组织引入了一种新的范式。更广泛地说,我们发现了新复制染色质的独特组织结构,这种组织结构必须通过活性过程重置,从而为表观遗传重编程提供基质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


