Stress disrupts engram ensembles in lateral amygdala to generalize threat memory in mice
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Stress induces aversive memory overgeneralization, a hallmark of many psychiatric disorders. Memories are encoded by a sparse ensemble of neurons active during an event (an engram ensemble). We examined the molecular and circuit processes mediating stress-induced threat memory overgeneralization in mice. Stress, acting via corticosterone, increased the density of engram ensembles supporting a threat memory in lateral amygdala, and this engram ensemble was reactivated by both specific and non-specific retrieval cues (generalized threat memory). Furthermore, we identified a critical role for endocannabinoids, acting retrogradely on parvalbumin-positive (PV+) lateral amygdala interneurons in the formation of a less-sparse engram and memory generalization induced by stress. Glucocorticoid receptor antagonists, endocannabinoid synthesis inhibitors, increasing PV+ neuronal activity, and knocking down cannabinoid receptors in lateral amygdala PV+ neurons restored threat memory specificity and a sparse engram in stressed mice. These findings offer insights into stress-induced memory alterations, providing potential therapeutic avenues for stress-related disorders.
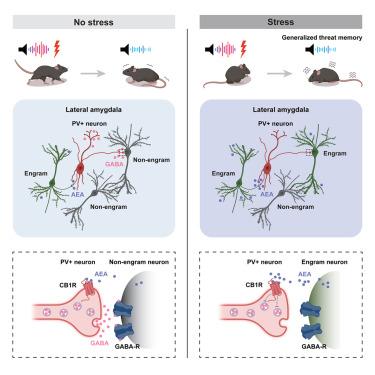
压力会破坏小鼠外侧杏仁核中的记忆组合,使威胁记忆泛化
压力会诱发厌恶性记忆过度泛化,这是许多精神疾病的特征。记忆是由在事件发生过程中活跃的稀疏神经元集合(engram集合)编码的。我们研究了介导压力诱导的小鼠威胁记忆过度泛化的分子和电路过程。通过皮质酮作用的应激增加了外侧杏仁核中支持威胁记忆的神经元群的密度,并且这种神经元群在特异性和非特异性检索线索(泛化威胁记忆)的作用下被重新激活。此外,我们还发现了内源性大麻素的关键作用,它逆向作用于蛛网膜旁腺素阳性(PV+)的外侧杏仁核中间神经元,在压力诱导下形成较稀疏的记忆片段和记忆泛化。糖皮质激素受体拮抗剂、内源性大麻素合成抑制剂、增加PV+神经元的活性以及敲除杏仁核外侧PV+神经元中的大麻素受体都能恢复应激小鼠的威胁记忆特异性和稀疏印记。这些发现深入揭示了应激诱导的记忆改变,为应激相关疾病提供了潜在的治疗途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


