Allogeneic chimeric antigen receptor cell therapies for cancer: progress made and remaining roadblocks
IF 81.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
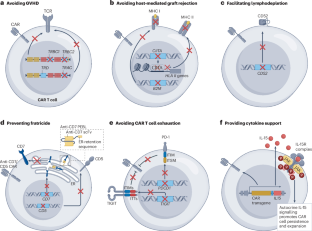
Chimeric antigen receptor (CAR) T cells are revolutionizing cancer therapy, particularly for haematological malignancies, conferring durable and sometimes curative responses in patients with advanced-stage disease. The CAR T cell products currently approved for clinical use are all autologous and are often effective; however, in patients who are lymphopenic and/or heavily pretreated with chemotherapy, autologous T cells can be difficult to harvest in sufficient numbers or have functional impairments that might ultimately render them less efficacious. Moreover, autologous products take several weeks to produce, and each product can be used in only one patient. By contrast, allogeneic CAR T cells can be produced for many patients using T cells from a single healthy donor, can be optimized for safety and efficacy, can be instantly available for ‘off-the-shelf’ use and, therefore, might also be more cost-effective. Despite these potential advantages, the development of allogeneic CAR T cells has lagged behind that of autologous products, owing to the additional challenges such as avoiding graft-versus-host disease and host-mediated graft rejection. Over the past few years, the development of advanced genome-editing techniques has facilitated the generation of novel allogeneic CAR T cell products. Furthermore, CAR cell products derived from other cell types such as induced pluripotent stem cells and natural killer cells are being investigated for clinical use. In this Review, we discuss the potential of allogeneic CAR cell products to expand life-saving immunotherapy to a much broader population of patients in the coming years, the progress made to date and strategies to overcome remaining hurdles. Several chimeric antigen receptor (CAR) T cell therapies are approved for the treatment of various haematological cancers; however, they are all autologous products requiring individualized manufacturing for each patient, which presents technical, logistical and resource challenges that limits scalability and implementation, and even raises certain ethical questions. Allogeneic products have the potential to address many of these issues, but come with their own challenges. This Review summarizes the advantages and disadvantages of allogeneic CAR cell products derived from T cells or other immune cells, their engineering and progress towards clinical implementation, as well as hurdles that remain to be overcome.

癌症的异体嵌合抗原受体细胞疗法:取得的进展和仍然存在的障碍
嵌合抗原受体(CAR)T 细胞正在彻底改变癌症治疗,尤其是血液恶性肿瘤的治疗,它能为晚期患者带来持久的、有时甚至是治愈性的治疗效果。目前获准用于临床的 CAR T 细胞产品都是自体细胞,而且通常都很有效;但是,对于淋巴细胞减少和/或接受大量化疗预处理的患者,自体 T 细胞可能很难获得足够的数量,或者存在功能障碍,最终可能导致疗效降低。此外,自体产品的生产需要数周时间,而且每种产品只能用于一名患者。相比之下,异体 CAR T 细胞可以使用来自单一健康供体的 T 细胞为许多患者生产,其安全性和有效性可以得到优化,可以立即 "现成 "使用,因此可能更具成本效益。尽管有这些潜在优势,但异体 CAR T 细胞的开发一直落后于自体产品,原因是要避免移植物抗宿主疾病和宿主介导的移植物排斥反应等额外挑战。过去几年中,先进基因组编辑技术的发展促进了新型异体 CAR T 细胞产品的产生。此外,从诱导多能干细胞和自然杀伤细胞等其他细胞类型中提取的 CAR 细胞产品也正被研究用于临床。在这篇综述中,我们将讨论异体 CAR 细胞产品在未来几年将拯救生命的免疫疗法扩展到更广泛患者群体的潜力、迄今为止取得的进展以及克服剩余障碍的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


