FMRP regulates MFF translation to locally direct mitochondrial fission in neurons
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
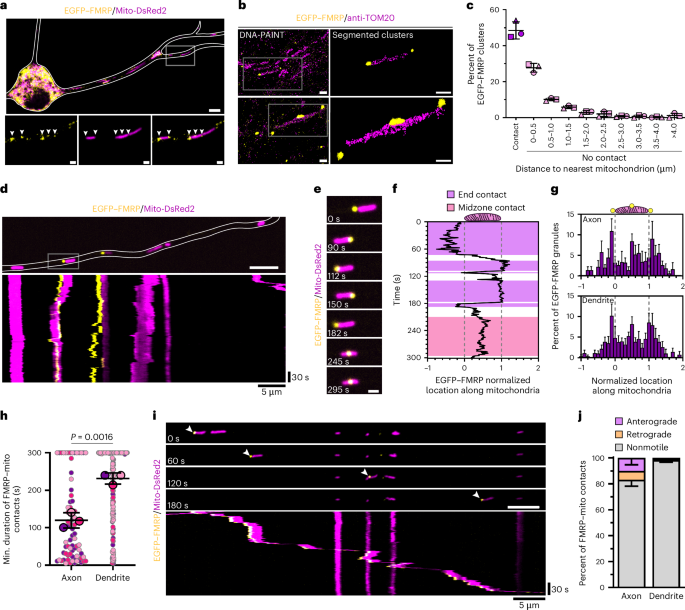
Fragile X messenger ribonucleoprotein (FMRP) is a critical regulator of translation, whose dysfunction causes fragile X syndrome. FMRP dysfunction disrupts mitochondrial health in neurons, but it is unclear how FMRP supports mitochondrial homoeostasis. Here we demonstrate that FMRP granules are recruited to the mitochondrial midzone, where they mark mitochondrial fission sites in axons and dendrites. Endolysosomal vesicles contribute to FMRP granule positioning around mitochondria and facilitate FMRP-associated fission via Rab7 GTP hydrolysis. Cryo-electron tomography and real-time translation imaging reveal that mitochondria-associated FMRP granules are ribosome-rich structures that serve as sites of local protein synthesis. Specifically, FMRP promotes local translation of mitochondrial fission factor (MFF), selectively enabling replicative fission at the mitochondrial midzone. Disrupting FMRP function dysregulates mitochondria-associated MFF translation and perturbs fission dynamics, resulting in increased peripheral fission and an irregular distribution of mitochondrial nucleoids. Thus, FMRP regulates local translation of MFF in neurons, enabling precise control of mitochondrial fission. Fenton et al. show that FMRP granules dock at the mitochondrial midzone in a Rab7-dependent manner in axons and dendrites, where they promote local MFF synthesis and fission at the mitochondrial midzone.

FMRP 调控 MFF 翻译,局部引导神经元线粒体分裂
脆性 X 信使核糖核蛋白(FMRP)是翻译的关键调节因子,其功能障碍会导致脆性 X 综合征。FMRP功能障碍会破坏神经元线粒体的健康,但目前还不清楚FMRP如何支持线粒体的平衡。在这里,我们证明了 FMRP 颗粒被招募到线粒体中区,并在那里标记轴突和树突中的线粒体裂变位点。溶酶体内囊泡有助于FMRP颗粒在线粒体周围的定位,并通过Rab7 GTP水解促进FMRP相关裂变。低温电子断层扫描和实时翻译成像显示,线粒体相关的FMRP颗粒是富含核糖体的结构,是局部蛋白质合成的场所。具体来说,FMRP 促进线粒体裂变因子(MFF)的局部翻译,有选择性地促成线粒体中区的复制裂变。干扰 FMRP 的功能会使线粒体相关 MFF 翻译失调并扰乱裂变动力学,导致外围裂变增加和线粒体核仁分布不规则。因此,FMRP 可调节神经元中 MFF 的局部翻译,从而实现对线粒体裂变的精确控制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


