Simultaneous deammoniation and denitrification under vacuum ultraviolet irradiation
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
The oxidative nitrate (NO3−) and reductive ammonium (NH4+) constitute the common nitrogen pollution in water. However, the high energy barrier of the comproportionation reaction makes it challenging for the deammoniation and denitrification reactions to occur simultaneously. This study evaluated the performance of simultaneous deammoniation and denitrification under vacuum ultraviolet (VUV) irradiation. The results demonstrate that the reduction reaction of NO3− and the oxidation reaction of NH4+ conform to the pseudo-first-order reaction kinetics, with respective kinetic constants of 0.012 min−1 and 0.002 min−1. The presence of Cl− and SO42− inhibits the reduction of NO3−, while HCO3− and CO32− promote NO3− reduction and NH4+ oxidation. The reaction mechanism of simultaneous NO3− and NH4+ removal was proven through free radical capture and electron spin resonance (ESR) experiments. It was determined that NO3− was reduced to NO2−, NH4+, and N2 by hydrated electrons (eaq–) in turn. While the free hydroxyl radical (·OH) produced by direct photolysis of water by VUV was identified as the key to the oxidation of NH4+. When NO3− and NH4+ coexist, they can capture eaq– and ·OH, respectively, thus avoiding the quenching of the two highly active species. Thus, energy waste is avoided and nitrogen removal efficiency is improved. The process study shows that the adjustment of the NO3− and NH4+ ratio and the control of process conditions are essential for the synchronous conversion of nitrate and ammonium.
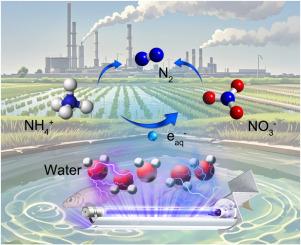
在真空紫外线辐照下同时进行脱氨和脱氮。
氧化型硝酸盐(NO3-)和还原型铵盐(NH4+)构成了水中常见的氮污染。然而,由于配比反应的能量障碍较高,因此同时进行脱氨和脱氮反应具有挑战性。本研究评估了在真空紫外线(VUV)照射下同时进行脱氨和脱硝反应的性能。结果表明,NO3- 的还原反应和 NH4+ 的氧化反应符合伪一阶反应动力学,动力学常数分别为 0.012 min-1 和 0.002 min-1。Cl- 和 SO42- 的存在抑制了 NO3- 的还原,而 HCO3- 和 CO32- 则促进了 NO3- 的还原和 NH4+ 的氧化。通过自由基捕获和电子自旋共振(ESR)实验证明了同时去除 NO3- 和 NH4+ 的反应机理。结果表明,NO3-依次被水合电子(eaq-)还原成 NO2-、NH4+ 和 N2。而紫外光直接光解水产生的自由羟基自由基(-OH)被认为是氧化 NH4+ 的关键。当 NO3- 和 NH4+ 共存时,它们可以分别捕获 eaq- 和 -OH,从而避免两种高活性物种的淬灭。因此,既避免了能源浪费,又提高了脱氮效率。工艺研究表明,调整 NO3- 和 NH4+ 的比例以及控制工艺条件对硝酸盐和铵的同步转化至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
文献相关原料
公司名称
产品信息
阿拉丁
Sodium persulfate (Na2S2O8)
阿拉丁
Sodium bicarbonate (NaHCO3)
阿拉丁
Sodium carbonate (Na2CO3)
阿拉丁
Sodium chloride (NaCl)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


