Efficient oxidative remediation of polycyclic aromatic hydrocarbons (PAHs)-contaminated soil: A thorough comprehension of Fe-loaded biochar activated persulfate
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
The porous and defective structure of biochar (BC) can accelerate surface electron transfer, promote the generation of more reactive oxygen species (ROS) by persulfate (PS), and effectively degrade organic pollutants in the soil. Electron transfer is a crucial link in this process, directly determining its oxidative degradation efficiency. In this study, using a novel strategy of enhancing electron transfer on the surface of BC by loading iron, three Fe-loaded BC activators (Fe-FeOx@BC, Fe2O3@BC and Fe3O4@BC) were synthesized to support the oxidative remediation of benzo(a)pyrene (BaP, Model compound of PAHs)-contaminated soil by PS. The results showed that Fe3O4@BC supported PS oxidation and remediation of BaP-contaminated soil had the best effect among the three BC-based activators, and the reuse effect was stable. Under the conditions of Fe3O4@BC addition of 1.00 wt%, PS addition of 0.75 wt%, reaction temperature of 35 °C, and solid-liquid ratio of 1:2.5, the removal rate of BaP in the soil reached the maximum of 93.84% at 120 min, and the soil toxicity was significantly reduced after remediation. The defect structure, conductive magnetic particles, and active functional groups on the surface of Fe3O4@BC were the key factors for activating PS to degrade BaP. With the combined action of the free radical pathway caused by ROS and the non-free radical pathway caused by 1O2, electron transfer, and active functional groups, BaP was degraded to small molecules such as CO2 and H2O, achieving rapid and efficient remediation of organic contaminated soil.
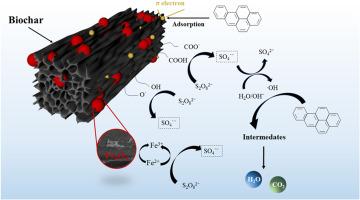
多环芳烃(PAHs)污染土壤的高效氧化修复:对含铁生物炭活化过硫酸盐的透彻理解。
生物炭(BC)的多孔缺陷结构可加速表面电子传递,促进过硫酸盐(PS)生成更多活性氧(ROS),有效降解土壤中的有机污染物。电子传递是这一过程中的关键环节,直接决定了其氧化降解效率。本研究采用一种通过负载铁来增强萃取剂表面电子传递的新策略,合成了三种负载铁的萃取剂活化剂(Fe-FeOx@BC、Fe2O3@BC 和 Fe3O4@BC),以支持 PS 对苯并(a)芘(BaP,多环芳烃的模式化合物)污染土壤的氧化修复。结果表明,Fe3O4@BC支持PS氧化修复BaP污染土壤的效果在三种BC基活化剂中最好,且重复利用效果稳定。在Fe3O4@BC添加量为1.00 wt%、PS添加量为0.75 wt%、反应温度为35 ℃、固液比为1:2.5的条件下,土壤中BaP的去除率在120 min时达到最大值93.84%,修复后土壤毒性明显降低。Fe3O4@BC表面的缺陷结构、导电磁性颗粒和活性官能团是激活PS降解BaP的关键因素。在 ROS 引起的自由基途径和 1O2 引起的非自由基途径、电子传递和活性官能团的共同作用下,BaP 被降解为 CO2 和 H2O 等小分子物质,实现了对有机污染土壤的快速高效修复。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


