Catalytic adsorption and decomposition of per(fluorinated) compounds using zeolites for greenhouse gas mitigation
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Per(fluorinated) compounds (PFCs/FCs) are a class of synthetic chemicals that are widely used in various industrial applications because of their unique properties. However, in recent years, their abundance in the environment has resulted in serious adverse effects on human health, raising crucial concerns in the environmental field. In this study, zeolites (Beta, Y, and ZSM-5) were used as catalysts for the decomposition and adsorption of PFCs/FCs (CF4, SF6, NF3, C3F8, and C4F8) by varying the temperature, pH, and contact time. Field emission scanning electron microscopy, X-ray diffraction, and Fourier transform infrared spectroscopy were employed to characterize the zeolite before and after the decomposition of the PFCs/FCs. A 100% complete decomposition of SF6 was observed when using ZSM-5, followed by a 75% and 45% decomposition when using Beta and Y zeolites, respectively. A 100% decomposition of NF3 was achieved by all zeolites (Beta, Y, and ZSM-5). ZSM-5 decomposed CF4, C3F8, and C4F8 to produce CO2 with the following removal rate: CF4 (80%) > C3F8 (60%) > C4F8 (25%). The decomposition of SF6 and CF4 produces SOF2, SOF4, SO2F2, and CO2, whereas that of NF3 yields SiF4 and NO. The reaction constants K of catalytic decomposition were calculated to be in the order: Y > Beta > ZSM-5. These results suggest that zeolite catalysts possess great potential as cost-effective and environmentally friendly catalysts for the decomposition of PFC/FC, thus reducing its adverse effects on the environment.
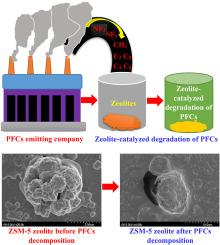
利用沸石催化吸附和分解全(氟)化合物,减少温室气体排放。
全氟化合物(PFCs/FCs)是一类合成化学品,因其独特的性质而被广泛应用于各种工业领域。然而,近年来,它们在环境中的大量存在对人类健康造成了严重的不良影响,引起了环境领域的高度关注。本研究以沸石(Beta、Y 和 ZSM-5)为催化剂,通过改变温度、pH 值和接触时间来分解和吸附 PFCs/FCs(CF4、SF6、NF3、C3F8 和 C4F8)。采用场发射扫描电子显微镜、X 射线衍射和傅立叶变换红外光谱对 PFCs/FCs 分解前后的沸石进行了表征。使用 ZSM-5 沸石时,观察到 SF6 100%完全分解;使用 Beta 和 Y 沸石时,分别观察到 75% 和 45% 的分解。所有沸石(Beta、Y 和 ZSM-5)都能 100% 分解 NF3。ZSM-5 分解 CF4、C3F8 和 C4F8 生成 CO2 的去除率如下:CF4(80%)> C3F8(60%)> C4F8(25%)。SF6 和 CF4 的分解产生 SOF2、SOF4、SO2F2 和 CO2,而 NF3 的分解则产生 SiF4 和 NO。经计算,催化分解的反应常数 K 顺序为:Y > Beta > ZSM-5:Y > Beta > ZSM-5。这些结果表明,沸石催化剂在分解全氟化合物/全氟化碳方面具有巨大的潜力,是一种经济、环保的催化剂,可减少全氟化合物/全氟化碳对环境的不利影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


