Uranium adsorption by iron modified zeolite and zeolite composite membranes
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Composite membranes incorporated with high-performance adsorbents are promising for uranium removal. The impact of speciation and ionic strength on uranium adsorption by zeolites was investigated in both static adsorption and composite membrane filtration. Zeolites with high Si/Al ratios exhibited the highest uranium adsorption capacity. Iron-modified zeolite, BEA-Fe30 completely removed uranium at a concentration of 0.6 g/L in static adsorption, with uranium uptake ranging from 125 to 130 μg/g at pH values between 6 and 12. At lower pH values, uptake decreased, dropping to 3 μg/g at pH 2. The increased uranium uptake between pH 6 and 12 is attributed to the formation of a ternary complex involving U(VI), carbonate, and Fe oxide surface (hydr)oxo sites. High ionic strength did not impact the adsorption of uranium. Additionally, PHREEQC modeling was employed to simulate uranium speciation and adsorption behavior under varying pH and ionic strength conditions, further validating experimental findings. Zeolite-loaded microfiltration/ultrafiltration (MF/UF) membranes achieved the WHO guideline of 30 μg/L uranium in the permeate, using less zeolite compared to static adsorption. With 0.25 g of zeolite, the MF/UF process achieved a uranium uptake of 699 μg/g, significantly higher than the 256 μg/g observed in static adsorption. However, uranium removal decreased with increased flow rates, suggesting mass transfer limitations during filtration. The study highlights the potential of composite membranes with high-performance zeolites for efficient uranium removal, contributing to advancements in water purification technologies and addressing environmental contamination.
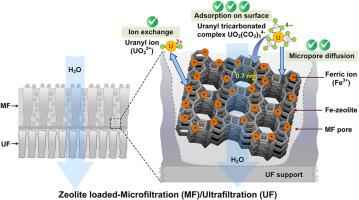
铁改性沸石和沸石复合膜对铀的吸附。
含有高性能吸附剂的复合膜在去除铀方面大有可为。在静态吸附和复合膜过滤中,研究了沸石对铀的吸附的种类和离子强度的影响。高硅/铝比率的沸石表现出最高的铀吸附能力。在静态吸附中,铁改性沸石 BEA-Fe30 可完全去除浓度为 0.6 克/升的铀,在 pH 值为 6 到 12 之间时,铀的吸收量为 125 到 130 微克/克。在 pH 值较低时,铀的吸收量下降,在 pH 值为 2 时降至 3 微克/克。 pH 值为 6 至 12 时铀的吸收量增加,这是因为形成了涉及铀(VI)、碳酸盐和氧化铁表面(氢)氧化位点的三元复合物。高离子强度并不影响铀的吸附。此外,还采用 PHREEQC 模型模拟了不同 pH 值和离子强度条件下的铀分化和吸附行为,进一步验证了实验结果。与静态吸附相比,沸石负载的微滤/超滤(MF/UF)膜用较少的沸石就达到了渗透物中铀含量为 30 μg/L 的世卫组织标准。在使用 0.25 克沸石的情况下,MF/UF 工艺的铀吸收量达到 699 微克/克,明显高于静态吸附工艺的 256 微克/克。然而,铀的去除率随着流速的增加而降低,这表明在过滤过程中存在传质限制。这项研究强调了带有高性能沸石的复合膜在高效去除铀方面的潜力,有助于推动水净化技术的进步和解决环境污染问题。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


