A multifunctional cascade gas-nanoreactor with MnO2 as a gatekeeper to enhance starvation therapy and provoke antitumor immune response
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Glucose oxidase (GOx)-mediated starvation therapy is an effective tumor treatment that blocks energy and activates the immune response. However, the insufficient tumor immunogenicity and immunosuppressive tumor microenvironment (TME) limited its therapeutic efficacy. To address this, we have designed a multifunctional cascade gas-nanoreactor with a MnO2 coating, which serves as an out gatekeeper to encapsulate both GOx and a carbon monoxide (CO) donor (denoted as GCM). Due to the protective effect of MnO2 coating, GCM maintains better stability in normal physiological environments, enhancing the catalytic activity of GOx and minimizing toxic side effects. Upon accumulation in the tumor, the degradation of MnO2 coating exposes the GOx enzyme, thereby initiating a cascade catalysis reaction to generate hydrogen peroxide (H2O2) and release CO in the hypoxic conditions. Additionally, the released Mn2+ reacts with H2O2 to generate toxic hydroxyl radical (•OH) as chemodynamic therapy (CDT). The synergistic treatments of starvation therapy, CO gas therapy and CDT effectively kill cancer cells and amplify immunogenic cell death (ICD), maturing DC cells and activating anti-tumor immune response. Furthermore, the released CO increases M1 macrophages infiltration and reduces myeloid-derived suppressor cells (MDSCs) infiltration, thus reversing the immunosuppressive TME. This multifunctional gas-nanoreactor provides a strategy for CO gas generation to trigger a robust anti-tumor immune response and has the potential for clinical application in cancer immunotherapy.
Statement of significance
A multifunctional cascade gas-nanoreactor with a MnO2 gatekeeper was developed to perform synergistic treatments involving starvation therapy, CO gas therapy and chemodynamic therapy (CDT) for tumor elimination. The MnO2 gatekeeper enhanced the catalytic activity of GOx within the nanoreactor by generating oxygen, thereby minimizing toxic side effects after intravenous injection. The gas-nanoreactor amplified ICD through synergistic treatments to mature DC cells and activate anti-tumor immune response. Furthermore, the released CO could reverse the immunosuppression of the TME to enhance cancer immunotherapy. The combination strategy utilizing the gas-nanoreactor demonstrates clinical potential for facilitating cancer immunotherapy.
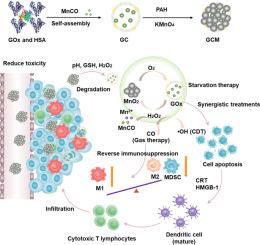
以 MnO2 为守门员的多功能级联气体纳米反应器可加强饥饿疗法并激发抗肿瘤免疫反应。
葡萄糖氧化酶(GOx)介导的饥饿疗法是一种有效的肿瘤治疗方法,它能阻断能量并激活免疫反应。然而,肿瘤免疫原性不足和免疫抑制性肿瘤微环境(TME)限制了其疗效。为了解决这个问题,我们设计了一种多功能级联气体纳米反应器,它具有 MnO2 涂层,可作为一个外置守门员,同时封装 GOx 和一氧化碳(CO)供体(称为 GCM)。由于 MnO2 涂层的保护作用,GCM 在正常生理环境中能保持较好的稳定性,从而提高 GOx 的催化活性,并将毒副作用降至最低。在肿瘤中积累后,MnO2 涂层降解会暴露出 GOx 酶,从而启动级联催化反应,在缺氧条件下生成过氧化氢(H2O2)并释放出 CO。此外,释放的 Mn2+ 与 H2O2 反应生成有毒的羟基自由基(-OH),这就是化学动力学疗法(CDT)。饥饿疗法、CO 气体疗法和 CDT 的协同治疗可有效杀死癌细胞并扩大免疫原性细胞死亡(ICD),使 DC 细胞成熟并激活抗肿瘤免疫反应。此外,释放的 CO 还能增加 M1 巨噬细胞的浸润,减少髓源性抑制细胞(MDSCs)的浸润,从而逆转免疫抑制的 TME。这种多功能气体纳米反应器提供了一种生成二氧化碳气体的策略,可触发强有力的抗肿瘤免疫反应,有望在癌症免疫疗法中得到临床应用。意义说明:该研究开发了一种带有 MnO2 守门装置的多功能级联气体纳米反应器,用于实施包括饥饿疗法、一氧化碳气体疗法和化学动力学疗法(CDT)的协同治疗,以消除肿瘤。MnO2 守门员通过产生氧气增强了纳米反应器内 GOx 的催化活性,从而将静脉注射后的毒副作用降至最低。气体纳米反应器通过协同处理使 DC 细胞成熟并激活抗肿瘤免疫反应,从而扩大了 ICD。此外,释放的 CO 可逆转 TME 的免疫抑制,从而增强癌症免疫疗法。利用气体纳米反应器的组合策略展示了促进癌症免疫疗法的临床潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
文献相关原料
公司名称
产品信息
上海源叶
Glucose oxidase
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


