Oxidative stress levels and antioxidant defense mechanisms (Nrf2-Keap1 signaling pathway) in the Harderian glands of hibernating Daurian ground squirrels
IF 1.9
3区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology
Pub Date : 2024-11-06
DOI:10.1016/j.cbpb.2024.111044
引用次数: 0
Abstract
Cyclic hibernation bouts in Daurian ground squirrels (Spermophilus dauricus) lead to repeated suppression and recovery of mitochondrial respiratory function across multiple organs, potentially impacting reactive oxygen species (ROS) dynamics. The Harderian gland (HG) plays an important role in endocrine regulation through porphyrin secretion. However, the influence of hibernation on oxidative pressure and associated antioxidant pathways in the HG remains inadequately understood. In the current study, we investigated the morphological changes, secretory activity, ROS levels, and underlying mechanisms in the HG of Daurian ground squirrels at distinct circannual stages of hibernation. Results indicated that: (1) Protoporphyrin levels in the HG increased during hibernation compared to the summer active (SA) phase, with a reduction in acinar lumen during torpor, potentially related to hibernation in a low-light environment. (2) Hydrogen peroxide (H2O2) and malondialdehyde (MDA) content during hibernation and post-hibernation (POST) did not exceed the levels observed in SA, indicating that the HG effectively mitigated oxidative pressure and lipid peroxidation during these periods. (3) Superoxide dismutase (SOD) activity increased while glutathione peroxidase (GPx) activity decreased during Inter-bout arousal (IBA) compared to both SA and torpor, although total antioxidant capacity (T-AOC) remained stable across all stages. (4) Overall fluorescent intensity of nuclear factor erythroid 2-related factor 2 (Nrf2) and Kelch-like ECH-associated protein 1 (Keap1) was significantly lower than in SA. These findings demonstrate that the HG in Daurian ground squirrels maintains a favorable oxidative status through the regulation of antioxidant enzyme activities during hibernation and even post-hibernation.
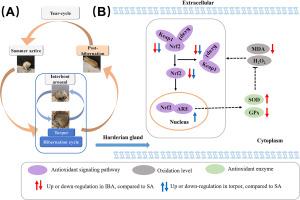
冬眠的达乌尔地松鼠哈氏腺的氧化应激水平和抗氧化防御机制(Nrf2-Keap1 信号通路)。
达乌尔地松鼠(Spermophilus dauricus)的周期性冬眠会导致多个器官线粒体呼吸功能的反复抑制和恢复,从而可能影响活性氧(ROS)的动态变化。哈德氏腺(HG)通过分泌卟啉在内分泌调节中发挥着重要作用。然而,人们对冬眠对哈德氏腺氧化压力和相关抗氧化途径的影响仍然了解不足。在本研究中,我们研究了达乌尔土松鼠在冬眠的不同循环阶段中HG的形态变化、分泌活性、ROS水平及其内在机制。结果表明(1) 与夏季活动期(SA)相比,冬眠期HG中的原卟啉水平升高,蛰伏期尖顶管腔缩小,这可能与低光照环境下的冬眠有关。(2)冬眠期和冬眠后(POST)的过氧化氢(H2O2)和丙二醛(MDA)含量没有超过在夏季活动期观察到的水平,这表明在这些时期,HG 有效地减轻了氧化压力和脂质过氧化反应。(3)尽管总抗氧化能力(T-AOC)在所有阶段都保持稳定,但与 SA 和冬眠期相比,冬眠间期(IBA)超氧化物歧化酶(SOD)活性增加,而谷胱甘肽过氧化物酶(GPx)活性降低。(4)核因子红细胞 2 相关因子 2(Nrf2)和 Kelch-like ECH-associated protein 1(Keap1)的总体荧光强度明显低于 SA 期。这些研究结果表明,达乌尔地鼠的HG在冬眠期间甚至冬眠后都能通过调节抗氧化酶的活性保持良好的氧化状态。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
4.60
自引率
4.50%
发文量
77
审稿时长
22 days
期刊介绍:
Comparative Biochemistry & Physiology (CBP) publishes papers in comparative, environmental and evolutionary physiology.
Part B: Biochemical and Molecular Biology (CBPB), focuses on biochemical physiology, primarily bioenergetics/energy metabolism, cell biology, cellular stress responses, enzymology, intermediary metabolism, macromolecular structure and function, gene regulation, evolutionary genetics. Most studies focus on biochemical or molecular analyses that have clear ramifications for physiological processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


