Screens for mutants defective in UapA trafficking highlight the importance of ER-exit as a primary control point in transporter biogenesis
IF 2.4
3区 生物学
Q3 GENETICS & HEREDITY
引用次数: 0
Abstract
Most transmembrane membrane proteins are thought to traffic to the plasma membrane (PM) via the conventional secretory pathway through sorting from the Golgi. However, our recent work has shown that in the filamentous fungus Aspergillus nidulans several nutrient transporters and other major membrane proteins traffic to the PM via Golgi-bypass and independently of known post-Golgi secretory mechanisms. Here in an effort to dissect the molecular mechanism underlying membrane cargo trafficking via Golgi-bypass we design and use unbiased genetic screens, based on the UapA uric acid-xanthine transporter, which allowed the isolation of mutants defective in UapA translocation to the plasma membrane. Analyses of these mutants highlight the importance of ER-exit as the primary control point in transporter trafficking via Golgi-bypass. Most mutants isolated concerned mutations within the uapA gene, albeit we also obtained uapA extragenetic mutants affecting secretion and growth pleiotropically or leading on apparent activation of an efflux transporter related to purine-detoxification. Our work paves the way to use genetic approaches targeting specifically trafficking mutations affecting Golgi-bypass.
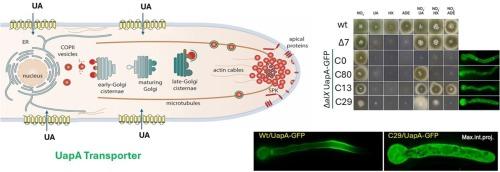
对 UapA 转运缺陷突变体的筛选突显了 ER 出口作为转运体生物发生过程中主要控制点的重要性。
大多数跨膜蛋白被认为是通过传统的分泌途径,从高尔基体分拣进入质膜(PM)的。然而,我们最近的研究表明,在丝状真菌黑曲霉(Aspergillus nidulans)中,几种营养物质转运体和其他主要膜蛋白通过高尔基体旁路(Golgi-bypass)转运到质膜(PM),与已知的高尔基体后分泌机制无关。在这里,为了剖析通过高尔基体旁路进行膜货物运输的分子机制,我们以 UapA 尿酸-黄嘌呤转运体为基础,设计并使用了无偏见的遗传筛选,从而分离出了 UapA 向质膜转运缺陷的突变体。对这些突变体的分析凸显了ER-出口作为通过高尔基体旁路进行转运的主要控制点的重要性。大多数分离出的突变体涉及 uapA 基因内部的突变,不过我们也获得了 uapA 基因外突变体,这些突变体会影响分泌和多向生长,或导致与嘌呤解毒有关的外排转运体的明显激活。我们的工作为使用遗传方法专门针对影响高尔基体旁路的转运突变铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fungal Genetics and Biology
生物-遗传学
CiteScore
6.20
自引率
3.30%
发文量
66
审稿时长
85 days
期刊介绍:
Fungal Genetics and Biology, formerly known as Experimental Mycology, publishes experimental investigations of fungi and their traditional allies that relate structure and function to growth, reproduction, morphogenesis, and differentiation. This journal especially welcomes studies of gene organization and expression and of developmental processes at the cellular, subcellular, and molecular levels. The journal also includes suitable experimental inquiries into fungal cytology, biochemistry, physiology, genetics, and phylogeny.
Fungal Genetics and Biology publishes basic research conducted by mycologists, cell biologists, biochemists, geneticists, and molecular biologists.
Research Areas include:
• Biochemistry
• Cytology
• Developmental biology
• Evolutionary biology
• Genetics
• Molecular biology
• Phylogeny
• Physiology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


