Jia Wei Qingxin Lotus Seed Drink ameliorates epithelial mesenchymal transition injury in diabetic kidney disease via inhibition of JMJD1C/SP1/ZEB1 signaling pathway
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Diabetic kidney disease (DKD) is one of the most common microvascular complications in patients with diabetes mellitus. In this condition, renal tubular epithelial mesenchymal transition (EMT) is an important factor accelerating the progression of DKD and a major cause of renal fibrosis and end-stage renal disease. However, the therapeutic effect is unsatisfactory because of the lack of effective drugs. Jia Wei Qingxin Lotus Seed Drink (QISD) is a traditional Chinese medicine compound formula that has shown to be effective in the clinical treatment of DKD. However, the potential of QISD in DKD-EMT treatment has yet to be fully explored.
Purpose
This study aimed to investigate the role of QISD in ameliorating DKD-EMT injury and its mechanism.
Methods
The active ingredients of QISD were identified via ultra-performance liquid chromatography-mass spectrometry/mass spectrometry (UHPLC-MS/MS). A DKD mouse model was constructed by high-fat diet feeding and intraperitoneal injection of STZ (60 mg/kg), and QISD (14.46, 28.92, and 57.84 g/kg/day) was administered by gavage for 12 consecutive weeks. Dapagliflozin (1 mg/kg/d) was used as a positive control. Renal pathological damage was observed by HE, PAS, and Masson staining. The expression levels of EMT-related proteins and pathway proteins were detected via immunohistochemistry, RT-qPCR, and western blot. In in vitro experiments, EMT injury was induced in human kidney tubular epithelial cells (HK-2) by using lipopolysaccharide (LPS). A combination of CCK8 assay, wound healing assay, small-molecule inhibitor intervention, and overexpression lentiviral transfection was used to investigate the effects of QISD on cell migration ability, adhesion ability, fibrotic factor formation, and mesenchymal properties.
Results
Animal experiments showed that QISD improved blood glucose, body weight, symptoms of excessive drinking and eating, and renal pathological injury in mice, reduced extracellular matrix deposition, delayed renal EMT injury, and inhibited the activation of the histone demethylase JMJD1C. UHPLC-MS/MS and molecular docking indicated that baicalin, wogonoside, oroxylin A-7-O-β-D-glucuronide, and glulisine A found in QISD could bind to JMJD1C. The ameliorating effect of QISD on DKD-EMT injury might be related to JMJD1C. The improvement of DKD-EMT injury by QISD was accompanied by the reduction of SP1 and ZEB1 expression. The SP1 overexpression not only reversed the therapeutic effect of JIB-04, an inhibitor of JMJD1C, on DKD-EMT but also exacerbated the expression of ZEB1 and downstream EMT-related factors. Thus, QISD might affect the expression of the epithelial marker E-cadherin by inhibiting the JMJD1C/SP1/ZEB1 signaling pathway, consequently preventing the transformation of epithelial cells to mesenchymal cells and ameliorating DKD-EMT injury.
Conclusion
This study was the first to demonstrate that QISD might ameliorate DKD-EMT injury by inhibiting the JMJD1C/SP1/ZEB1 signaling pathway. These findings provide strong pharmacologic evidence for the clinical use of QISD in the treatment of DKD.
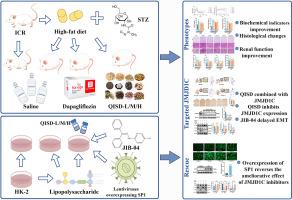
加味清心莲子饮通过抑制JMJD1C/SP1/ZEB1信号通路改善糖尿病肾病的上皮间充质转化损伤
背景:糖尿病肾病(DKD)是糖尿病患者最常见的微血管并发症之一:糖尿病肾病(DKD)是糖尿病患者最常见的微血管并发症之一。在这种情况下,肾小管上皮间充质转化(EMT)是加速 DKD 进展的重要因素,也是导致肾纤维化和终末期肾病的主要原因。然而,由于缺乏有效的药物,治疗效果并不理想。加味清心莲子饮(QISD)是一种中药复方制剂,在临床上治疗 DKD 有一定疗效。目的:本研究旨在探讨加味清心莲子饮在改善DKD-EMT损伤中的作用及其机制:方法:通过超高效液相色谱-质谱/质谱联用技术(UHPLC-MS/MS)鉴定喹乙醇的有效成分。通过高脂饮食喂养和腹腔注射 STZ(60 毫克/千克)构建 DKD 小鼠模型,连续 12 周灌胃给药 QISD(14.46、28.92 和 57.84 克/千克/天)。达帕格列净(1 毫克/千克/天)作为阳性对照。通过HE、PAS和Masson染色观察肾脏病理损伤。通过免疫组化、RT-qPCR和Western印迹检测EMT相关蛋白和通路蛋白的表达水平。在体外实验中,使用脂多糖(LPS)诱导人肾小管上皮细胞(HK-2)的 EMT 损伤。结合CCK8检测、伤口愈合检测、小分子抑制剂干预和过表达慢病毒转染等方法,研究了QISD对细胞迁移能力、粘附能力、纤维化因子形成和间质特性的影响:动物实验表明,QISD能改善小鼠血糖、体重、多饮多食症状和肾脏病理损伤,减少细胞外基质沉积,延缓肾脏EMT损伤,抑制组蛋白去甲基化酶JMJD1C的活化。超高效液相色谱-质谱/质谱(UHPLC-MS/MS)和分子对接表明,芪冬皂甙中的黄芩苷、乌药苷、大黄素 A-7-O-β-D-葡萄糖醛酸苷和谷维素 A 能与 JMJD1C 结合。QISD 对 DKD-EMT 损伤的改善作用可能与 JMJD1C 有关。QISD 对 DKD-EMT 损伤的改善伴随着 SP1 和 ZEB1 表达的减少。SP1的过表达不仅逆转了JMJD1C抑制剂JIB-04对DKD-EMT的治疗效果,还加剧了ZEB1及下游EMT相关因子的表达。因此,QISD可能通过抑制JMJD1C/SP1/ZEB1信号通路影响上皮标志物E-cadherin的表达,从而阻止上皮细胞向间充质细胞转化,改善DKD-EMT损伤:本研究首次证明了 QISD 可通过抑制 JMJD1C/SP1/ZEB1 信号通路来改善 DKD-EMT 损伤。这些发现为 QISD 在临床上用于治疗 DKD 提供了有力的药理学证据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


