Disruption of local circadian clocks in aristolochic acid-induced nephropathy in mice
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
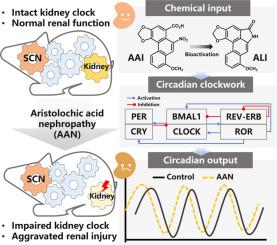
Aristolochic acid I (AAI), an emerging biogenic contaminant widely present in Aristolochic plants, has been implicated in the progression of tubulointerstitial disease, known as aristolochic acid nephropathy (AAN). The circadian clock, a vital regulator of organ homeostasis, is susceptible to external chemical cues, including toxins. However, the reciprocal interactions between AAI and the circadian clock remain unexplored.
Methods
We initially assessed sex- and time-dependent nephropathy and behavioral responses in C57BL/6J mice exposed to AAI. Subsequently, we evaluated changes in the expression of circadian clock genes following treatment with AAI or its bioactive metabolite, aristolactam I, using real-time quantitative PCR and immunoblotting in renal tissues and cells. Additionally, real-time reporter assays were conducted on kidney explants from PER2::Luc knock-in reporter mice and Per2-dLuc/Bmal1-dLuc reporter cell lines. To further elucidate the regulatory role of circadian clocks in AAI-induced nephropathy, mice with global or kidney-specific knockout of Bmal1, as well as mice subjected to experimental jetlag, were utilized.
Results
Our findings revealed a sex-dependent nephrotoxicity of AAI, with males exhibiting greater vulnerability. AAI-induced nephropathy was accompanied by impaired spatial cognitive function, disruptions in free-running locomotor activity, altered renal expression of multiple core clock genes, and disturbances in the circadian rhythm of renal PER2::Luc activity. Notably, kidney-specific ablation of the core clock gene Bmal1 significantly exacerbated renal injury and inflammation, whereas disruptions to the central clock, either genetically (through conventional knockout of Bmal1) or environmentally (mimicking jetlag), had minimal effects on AAI nephrotoxicity. Furthermore, both AAI and its bioactive metabolite aristolactam I demonstrated the ability to disrupt circadian clocks in human osteosarcoma cells (U2OS) and mouse renal tubular epithelial cells (mRTEC).
Conclusion
Collectively, these findings highlight the detrimental impact of aristolochic acids on local renal circadian clocks, ultimately exacerbating kidney damage. This study provides novel insights into the molecular mechanisms underlying AAI nephrotoxicity, potentially opening avenues for therapeutic interventions aimed at modulating the renal circadian clock to treat AAN.
马兜铃酸诱发小鼠肾病的局部昼夜节律紊乱
背景:马兜铃酸 I(AAI)是一种广泛存在于马兜铃科植物中的新兴生物污染物,与肾小管间质疾病(即马兜铃酸肾病,AAN)的发展有关。昼夜节律钟是器官平衡的重要调节器,易受包括毒素在内的外部化学线索的影响。然而,AAI与昼夜节律钟之间的相互影响仍未得到研究:我们首先评估了暴露于 AAI 的 C57BL/6J 小鼠肾病和行为反应的性别和时间依赖性。随后,我们在肾组织和细胞中使用实时定量 PCR 和免疫印迹法评估了 AAI 或其生物活性代谢物马兜铃内酰胺 I 治疗后昼夜节律钟基因表达的变化。此外,还对来自 PER2::Luc 基因敲入报告小鼠和 Per2-dLuc/Bmal1-dLuc 报告细胞系的肾脏外植体进行了实时报告分析。为了进一步阐明昼夜节律钟在 AAI 诱导的肾病中的调控作用,我们利用了 Bmal1 基因全面或肾脏特异性敲除的小鼠以及实验性时差小鼠:结果:我们的研究结果表明,AAI的肾毒性与性别有关,雄性小鼠更容易受到影响。AAI诱导的肾病伴随着空间认知功能受损、自由奔跑运动活动紊乱、多个核心时钟基因的肾脏表达改变以及肾脏PER2::Luc活动的昼夜节律紊乱。值得注意的是,肾脏特异性消减核心时钟基因 Bmal1 会显著加剧肾脏损伤和炎症,而通过基因(传统的 Bmal1 基因敲除)或环境(模拟时差)破坏中央时钟对 AAI 肾毒性的影响微乎其微。此外,AAI及其生物活性代谢物马兜铃内酰胺I都能破坏人骨肉瘤细胞(U2OS)和小鼠肾小管上皮细胞(mRTEC)的昼夜节律:总之,这些发现凸显了马兜铃酸对局部肾脏昼夜节律的有害影响,最终会加剧肾脏损伤。这项研究为了解 AAI 肾毒性的分子机制提供了新的视角,有可能为旨在调节肾昼夜节律以治疗 AAN 的治疗干预开辟道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


